Abstract
Background
To investigate the efficacy, safety, and patency following treatment of wall-adherent thrombus in hemodialysis vascular access with a wall-contact device, the Arrow-Trerotola percutaneous thrombolytic device (PTD).
Methods
We retrospectively reviewed an existing database of procedures fulfilling the following criteria: thrombosed hemodialysis access, wall-adherent thrombus, and use of PTD for mechanical thrombectomy. Data on immediate success, complications, and patency were collected from medical records, dialysis records, and angiographic reports.
Results
Ninety-three patients with 108 episodes of vascular access thrombosis were included in the study. Fifty-three of the procedures were performed on native fistulas, and 55 were on synthetic grafts. Anatomical and clinical success was achieved in 97% and 96% of the procedures, respectively. The average procedure time was 52 ± 23 minutes. Complications occurred in three of the procedures (2.7%), but none of these complications were device-related. The primary patency rates in the native fistula group were 57% and 42% at three and six months, respectively. The primary patency rates in the synthetic graft group were 40% at three months, and 27% at six months. The secondary patency rates at six months were 91% in the native fistula group, and 93% in the synthetic graft group.
Conclusions
Our results show that a wall-contact mechanical device, PTD, is effective and safe for endovascular removal of wall-adherent thrombi in hemodialysis vascular access in both native fistulas and synthetic grafts.
Keywords: Adherent, Angioplasty, Endovascular, Hemodialysis, Thrombectomy, Vascular access
INTRODUCTION
For patients undergoing hemodialysis, vascular access thrombosis remains a major contributor to morbidity and hospitalization.1,2 Traditionally, clotted vascular accesses have been salvaged by surgical thrombectomy: incision of the graft with a Fogarty balloon thrombectomy catheter and revision as necessary. In the past two decades, a growing number of endovascular techniques have been introduced to dissolve, fragment, macerate, or aspirate thrombus from the occluded access, including local infusion of thrombolytics, balloon declotting, catheter aspiration, and the use of mechanical devices. Such techniques have produced equivalent results to that of surgery.3,4
Among these endovascular methods, percutaneous mechanical devices have emerged as a means of rapidly cleaning clots from the occluded vessel while eliminating the need for thrombolytics. Mechanical devices, either using a wall-contact mechanism or a non wall-contact mechanism, are effective for debulking fresh thrombus.5 However, a substantial portion of patients may present late for a thrombectomy attempt. Thrombi in these late presentations may have become organized, fibrotic and tightly adherent to the vessel wall, making them resistant to balloon declotting or catheter aspiration. Currently, only one study has specifically evaluated the efficacy of mechanical devices for the removal of wall-adherent thrombus.6 In addition, the effect of wall-contact mechanical devices has not been reported in Taiwan or in other Asian populations. Accordingly, the aim of our study was to report our single-center experience of using a wall-contact device, the Arrow-Trerotola percutaneous thrombolytic device (PTD), to remove wall-adherent thrombi in hemodialysis vascular access.
MATERIALS AND METHODS
Study design
We performed a retrospective study from July 2010 to June 2012 using an existing database in our institution. Ethics approval was not required from our institutional review board for this type of retrospective study, but written informed consent was obtained from each patient receiving the thrombectomy procedures as well as other transluminal angioplasty procedures. Data on procedures collected from the hospital database fulfilled the following inclusion criteria: (1) thrombosed vascular access, (2) wall-adherent thrombus, and (3) PTD used for thrombectomy. In our hospital and nearby hemodialysis centers, patients with suspected vascular access thrombosis are first referred to our angiographic unit for endovascular salvage rather than surgical thrombectomy. A conventional percutaneous salvage technique using balloon maceration and sheath aspiration is first attempted. In our institution, mechanical thrombectomy devices are used only for vascular access with a large clot burden (with a risk of significant pulmonary embolism) or wall-adherent thrombus (causing compromised flow or anatomical failure). Wall-adherent thrombus was defined as thrombus that was resistant to repeated balloon maceration and sheath aspiration. Declotting was attempted only in instances of compromised blood flow or a stenosis diameter of more than 30%. All procedures fulfilling the above criteria were included on an intention-to-treat basis. Demographic data, access characteristics, and procedure details and follow-up data were obtained from medical records, angiography and angioplasty reports, and hemodialysis records.
Endovascular techniques
For nine patients without total thrombosis of vascular access, a standard angioplasty technique was applied through a patent segment of the access. The remaining 99 patients with total thrombosed access were salvaged by the modified Beathard’s “double sheath technique” in that arterial thrombectomy is performed first.7 The procedure was initiated by placing two 7-F sheaths (Terumo, Tokyo, Japan) within the access: the first one near the venous anastomosis or a patent vein segment directed toward the arterial inflow; the second one was placed near the arterial anastomosis directed toward the venous outflow. After successfully traversing the occluded segment and crossing the arterial anastomosis, the arterial plug was then dislodged by forceful withdrawal of the inflated (at 2 atm) balloon catheter from the arterial anastomosis to the venous sheath. Thrombi dislodged within the access segment were repeatedly aspirated via the sheath as much as possible. After forceful pulsation was achieved, the same procedure was repeated via the second sheath, directed toward the venous anastomosis, to remove the thrombus from the outflow vein to the arterial sheath. Only after the clots were removed was the venous stenosis dilated by standard balloon angioplasty, and a peripheral cutting balloon was reserved for lesions that were only amenable to high pressure balloon dilatation.8,9 The sheath was removed and the puncture site was compressed manually until hemostasis was obtained. As a general rule, heparin was not given during the procedure, except for patients with large thrombi, prolonged procedure time, or sheath to be left for dialysis after thrombectomy. After cessation of the procedure, an anti-platelet agent with aspirin or clopidogrel was given routinely for three days.
Mechanical thrombectomy for persistent thrombus
When wall-adherent thrombus persisted after repeated passes of balloon dilatation and maceration, a mechanical device was needed to restore blood flow or achieve anatomical success. During the study period, PTD was the only mechanical thrombectomy device available in our institution. The standard 5-F version PTD is not an over-the-wire device and is introduced into the clotted access through a sheath at least 5-F in diameter10 (Figure 1). The device was advanced through the thrombus in the closed position and the cage deployed by retracting the catheter. The rotator unit was activated to spin the cage, which was then pulled slowly through the adherent thrombus, fragmenting and stripping thrombus against the access walls. The resulting slurry was aspirated from the sheath after each pass of the device. As many passes as necessary were made to remove as much thrombus as possible. External manual compression was applied as needed to bring thrombus into contact with the device when working in large veins or aneurysmal segments. After the thrombectomy procedure, balloon dilatation was used as needed for residual stenosis or luminal irregularity. The over-the-wire (OTW) PTD is passed through a sheath at least 7-F diameter and tracks over a 0.025-inch guide wire. Its use is otherwise identical to that of the standard device (Figure 2). The OTW device has been approved by the U.S. Food and Drug Administration for use in native fistulas, and use of the standard device was off-label.
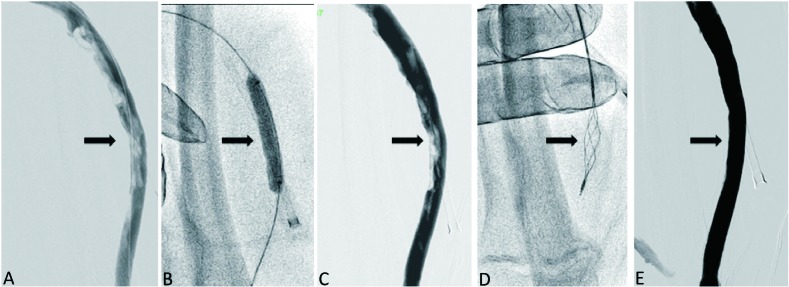
Figure 1.
Thrombectomy in an intra-graft segment of a thrombosed loop dialysis graft with a standard 5-F version Arrow-Trerotola percutaneous thrombectomy device (PTD). (A) Angiogram showed thrombi in the intra-graft segment. (B) Balloon dilatation, maceration and sheath aspiration were performed. (C) Residual wall-adherent thrombi persisted. (D) PTD was advanced, crossed and entrapped the thrombi, and then was rotated to strap the thrombi out the sheath. (E) Final angiogram showed no residual thrombus in the graft.
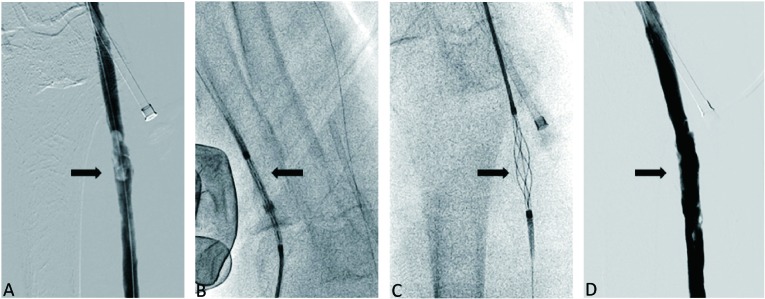
Figure 2.
Thrombectomy in a native outflow cephalic vein segment of a thrombosed loop dialysis graft with an over-the-wire 7-F version Arrow-Trerotola percutaneous thrombectomy device (PTD). (A) After balloon dilatation and maceration, wall-adherent thrombi persisted in the outflow cephalic vein. (B) The PTD was advanced via the arterial sheath via a 0.025-inch guide wire in the closed position. (C) After crossing the thrombi, the cage was deployed to entrap the thrombi and was rotated by a motor-driven unit, and then was pulled back into the sheath. (D) The final angiogram showed minimal residual thrombus.
Follow-up after thrombectomy
The patients from our institution were followed at our hemodialysis units. The patients from outside satellite hemodialysis centers were referred to our angiographic unit based on the following criteria: decreased or absent thrill, increased pulsation, development of collateral veins, limb swelling, difficulty in cannulation, prolonged bleeding after hemodialysis, high venous pressure during dialysis (dynamic venous pressure exceeding threshold level three consecutive times), decreased dialysis flow rate (total fistula blood flow less than 500 ml/min or a 25% reduction in blood flow from that at baseline), and an abnormal recirculation measurement (> 10% using the urea-based method). When abnormal clinical or hemodynamic parameters suggested fistula dysfunction, patients were referred for repeat fistulography and intervention as appropriate.
Definitions and statistics
Based on the reporting standard for thrombosed vascular access from the guidelines of the Society of Interventional Radiology (SIR), the following definitions were used in our study.11 Anatomical success was defined as restoration of flow combined with less than 30% maximal residual diameter stenosis for any significant underlying stenosis. Clinical success was defined as the ability to resume normal dialysis at least once after the intervention. Procedure time was defined as the time interval from puncture to the final angiogram. Post-interventional primary patency was defined as the interval from the intervention until the next access thrombosis or repeat intervention. Post-interventional secondary patency was the interval from the intervention until the access was surgically declotted, revised, or abandoned. Complications were classified as major or minor in accordance with published recommendations.11 Major complications resulted in hospital admission, unplanned increase in the level of care, prolonged hospitalization, permanent adverse sequelae or death. Minor complications resulted in no sequelae, nominal therapy, or short hospital stay for observation. In the analysis, each vascular access thrombosis was treated as a separate event for patients with more than one episode of thrombosis. Kaplan-Meier analyses were used to estimate primary and secondary patency rates. Statistical analyses were performed using STATISTICA 8.0 software for Windows (StatSoft Inc., Tulsa, OK, USA).
RESULTS
From July 2010 through June 2012, 108 endovascular procedures using PTD for mechanical thrombectomy of wall-adherent thrombus were performed on 93 patients (57 women and 36 men). Patient ages ranged from 24 to 88 years (mean, 63.9 years). The vascular accesses included 46 native fistulas and 47 synthetic grafts; 78 forearm accesses and 15 upper-arm accesses; 69 left-sided accesses and 24 right-sided accesses (Table 1). Fourteen patients underwent more than one thrombectomy procedure due to recurrent thrombosis. In all the procedures, PTD was used when wall-adherent thrombus remained after repeated balloon maceration or catheter aspiration. Adjuvant pharmacological thrombolysis, cutting balloon, or endovascular stenting were not used in any of the procedures. Heparin was administered during 70 of the procedures (64.8%).
Table 1. Characteristics of patients and vascular access .
| Total | Native | Graft | |
| (N = 93) | (N = 46) | (N = 47) | |
| Patients | |||
| Age (years/old) | 63.9 ± 13.0 | 63.5 ± 11.4 | 64.3 ± 14.6 |
| Female | 61.3% | 56.5% | 66.0% |
| Diabetes mellitus | 54.8% | 52.2% | 57.4% |
| Hypertension | 59.1% | 52.2% | 66.0% |
| Vascular access | |||
| Shunt age (months) | 51.6 ± 44.4 | 62.8 ± 50.8 | 40.4 ± 33.9 |
| Side (left side access) | 74.2% | 76.1% | 72.3% |
| Type (forearm access) | 83.9% | 89.1% | 78.7% |
| Total thrombosis | 91.3% | 90.0% | 92.5% |
Values are n (%) or mean ± SD.
Immediate outcome
The average procedure time was 52.4 ± 23.2 minutes; 61 minutes for native fistulas and 44 minutes for synthetic grafts. Anatomical success was achieved in 105 of the 108 thrombectomy procedures (97.2%). Only one patient experienced re-thrombosis before the next dialysis session, and clinical success was achieved in 104 of 108 procedures (96.3%). The causes of failure were failure of clot removal in two procedures in native fistulas and venous rupture during balloon dilatation in the native outflow vein of a graft. Consequently, anatomical success and clinical success rates were 96.2% and 96.2% in native fistulas and 98.1% and 96.3% in synthetic grafts, respectively.
Patency outcomes
Overall primary patency rates were 74.1%, 48.1%, and 34.3% and overall secondary patency rates were 95.4%, 93.5%, and 91.7% at 30, 90, and 180 days, respectively (Table 2). Procedures on native fistulas resulted in primary patency rates of 86.8%, 56.6% and 41.5% and secondary patency rates of 96.2%, 94.3%, and 90.6% at 30, 90, and 180 days, respectively (Table 2 and Figure 3). Procedures on synthetic grafts resulted in primary patency rates of 61.8%, 40.0% and 27.3% and secondary patency rates of 94.5%, 92.7%, and 92.7% at 30, 90, and 180 days, respectively (Table 2 and Figure 4).
Table 2. Success, patency and complication rates .
| Total | Native | Graft | |
| (N = 108) | (N = 53) | (N = 55) | |
| Procedure time (min) | 52.4 ± 23.2 | 61.6 ± 21.4 | 44.1 ± 21.8 |
| Success rates (%) | |||
| Anatomical success | 97.2 | 96.2 | 98.1 |
| Clinical success | 96.3 | 96.2 | 96.3 |
| Primary patency (%) | |||
| 30 days | 74.1 | 86.8 | 61.8 |
| 90 days | 48.1 | 56.6 | 40.0 |
| 180 days | 34.3 | 41.5 | 27.3 |
| Secondary patency (%) | |||
| 30 days | 95.4 | 96.2 | 94.5 |
| 90 days | 93.5 | 94.3 | 92.7 |
| 180 days | 91.7 | 90.6 | 92.7 |
| Complication rates (%) | |||
| Major | 0.9 | 1.8 | 0 |
| Minor | 1.8 | 3.7 | 0 |
Values are percentages or mean ± SD.
Figure 3.
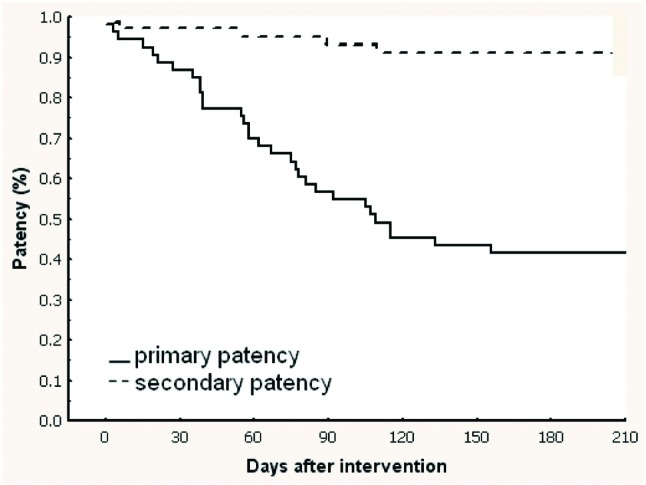
Kaplan-Meier curves showing post-interventional primary and secondary patency rates following procedures on native fistulas.
Figure 4.
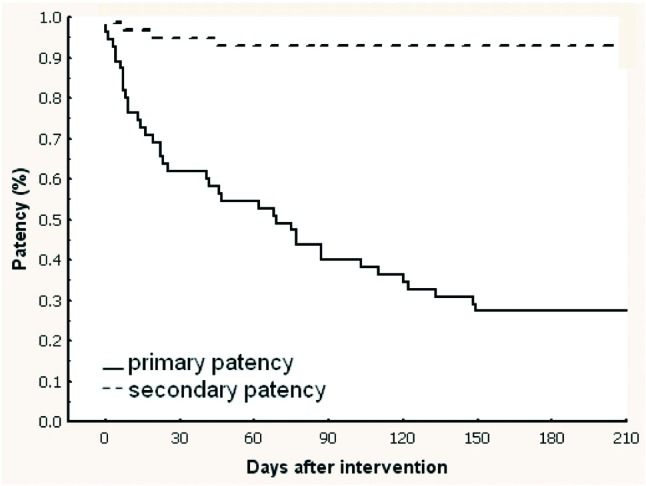
Kaplan-Meier curves showing post-interventional primary and secondary patency rates following procedures on synthetic grafts.
Complications
Three (2.7%) complications occurred in the 108 thrombectomy procedures: one arterial embolism, one extravasation and one venous rupture. The venous rupture was due to balloon dilatation rather than the thrombectomy device. The arterial embolism was successfully rescued by balloon embolectomy. Finally, the venous rupture was unable to be rescued because balloon tamponade failed to seal the venous rupture with a resulting hematoma. The procedure was abandoned with recurrence of thrombosis. No episodes of symptomatic pulmonary embolism were observed following thrombectomy.
DISCUSSION
Main findings
Our study demonstrated that a mechanical device with a wall-contact mechanism is effective and safe in the removal of wall-adherent thrombus in hemodialysis vascular access, in both synthetic grafts and native fistulas. The secondary patency rates were high and the primary patency rates were comparable to guideline recommendations.12
Clinical significance of wall-adherent thrombus
In the management of vascular access thrombosis, residual clots are a much greater problem than in interventions in coronary or peripheral arteries, regardless of whether surgical or endovascular thrombectomy is used. These thrombi can remain against the vessel wall after crushing or maceration by Fogarty balloons or dilatation balloons. In an angioscopic study evaluating the effect of percutaneous thrombectomy, a much greater amount of residual thrombus was found than revealed by angiography.13 The ultimate destiny of wall-adherent thrombi remains unknown. They may be washed away into the pulmonary circulation when blood flow is restored. Adherent thrombi that are not washed away may be gradually degraded by the patient’s fibrinolytic system or incorporated into the endoluminal surface. Bech et al. stress that residual wall-adherent thrombus after Fogarty balloon procedures are frequent and create an irregular surface. They constitute a source of turbulence and platelet aggregation, thereby facilitating re-thrombosis.14 However, the impact of these wall-adherent thrombi has not been proven in clinical studies.
Methods to remove wall-adherent thrombus
Before the development of mechanical devices, thrombolytic agents, balloon techniques and aspiration catheters were the only means of removing wall-adherent thrombus. However, a significant portion of thrombus may remain within the access despite use of these modalities, especially in hemodialysis vascular accesses. One reason is that the size of the catheters is relatively small compared to the diameter of the vascular access. In addition, thrombectomy may be delayed for days to weeks as dialysis can be continued via a temporary catheter or a collateral vein. These thrombi then became organized, fibrotic and incorporated into the vessel wall, making them resistant to crushing or aspiration. A variety of mechanical devices have been developed to debulk fresh thrombus, either via a non wall-contact mechanism (such as the AngioJet system) or a wall-contact mechanism (such as the PTD).5 In our review of the literature, only one mechanical device, the “Mesh Basket”, has been evaluated specifically in regard to wall-adherent thrombus.6 PTD is a relatively simple procedure that can be performed by any interventionist experienced with endovascular therapy of hemodialysis vascular access. In our institution, a practitioner could independently operate this device after less than five supervised PTD-using procedures.
In Taiwan, only two types of mechanical thrombectomy devices are available: the PTD catheter and the AngioJet system. The PTD catheter operates by a basket that is expanded to be in contact with the vessel wall. After trapping the thrombus in the basket, rapid rotation of the PTD up to 3,000 rpm will fragment the thrombus and strip the thrombus from the vessel wall. The stripping action of PTD can be further enhanced by manual compression, resulting in better contact with the thrombus. The efficacy of wall-contact devices is supported by an angioscopic study showing that wall-contact devices left less residual thrombus than hydrodynamic devices.15
Initial success
Only one small study has specifically evaluated the feasibility of a mechanical device (mesh-basket) for declotting wall-adherent thrombi in vascular access.6 Most studies evaluating mechanical devices have included procedures performed on both fresh thrombus and wall-adherent thrombus.3,4 Therefore, the characteristics of clots in such studies are different from those in our current study. Our technique success rate is the highest among available declotting techniques, which can range from 73-95%.3 Our success rate for native fistulas is even higher than previous studies, which have varied from 73-79%.4
For synthetic grafts, Dolmatch et al. reported a procedure time of 72 min using a Cragg thrombolytic brush in combination with urokinase, and Trerotola reported a procedure time of 75 minutes using the PTD.10,16 The procedure times in the above studies were more time-saving than devices using an aspiration mechanism, such as the aspiration catheter (1.5-2.5 hours, Turmel-Rodriguez et al.) or the AngioJet system (82 min, Wu et al).17-19 According to an analysis of a large body of data from the USA, the average procedure time for fistula declotting was 88 ± 42 minutes, which is significantly longer than that for grafts.20 Only patients with wall-adherent thrombus were selected in our study, which differs from previous studies where variable presentations of thrombosis have been included.
Safety
One of the concerns regarding wall-contact devices is the risk of injury to the vessel wall. Previous studies using PTD have demonstrated a comparable local complication rate to non wall-contact devices.8 The results of our study confirm the safety of wall-contact devices in clearing wall-adherent thrombus, both in synthetic grafts and native vessels. More importantly, the complications of vessel rupture and extravasation that occurred were not directly related to the device, but rather were due to balloon dilatation or wiring. Whether the observed complication of arterial embolism was related to the PTD is not clear, as multiple devices were passed through the anastomosis during the procedure. Another safety concern is the risk of acute pulmonary embolism. In our study, no patients experienced symptomatic acute pulmonary embolism. Similarly, acute symptomatic embolism has rarely been reported in previous studies of mechanical thrombectomy, either in grafts or native vascular access.21
The long term cardiopulmonary safety of endovascular mechanical thrombectomy has been proven by a retrospective study.22 Another concern regarding long-term effects is the interaction between the device and the vessel wall. The wall contact-feature and stripping action of PTD may cause endothelial injury, leading to an unfavorable impact on patency. Nonetheless, PTD has been shown to be no more injurious than a Fogarty catheter in terms of intimal injury in animal models.23,24 In addition, a recent study has shown that long-term patency rates did not differ between thrombectomy devices using a wall-contact mechanism and those using a non wall-contact mechanism.25
Patency
The patency rates in our series are within the range of previous reports for the percutaneous salvage of thrombosed native fistulas or synthetic grafts. Our 6-month primary and secondary patency rates for native fistulas are comparable to previous studies.26-28 Our three-month patency rate meets the recommended threshold provided by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI) guidelines.12 These results echo the common belief that patency after therapy of thrombosed vascular access is more dependent on the subsequent intervention required to treat the inciting lesion rather than the method of thrombectomy used. In addition, our results provide evidence that counters the concern that endothelial injury from wall-contact devices may cause an unfavorable impact on patency outcomes.
Limitation
The primary limitation of our study is its retrospective nature. Second, the number of patients in our study was relatively small. Third, follow-up in this series may not be perfect because of its retrospective nature. Fourth, asymptomatic pulmonary embolism after thrombectomy procedures may not be easily found. And finally, in contrast to residual stenosis and intra-graft pressure gradient, the impact of wall-adherent thrombi on the outcome of vascular access remains unknown.29
CONCLUSION
Endovascular declotting of wall-adherent thrombus with a wall-contact device is effective and safe for the treatment of thrombosed hemodialysis vascular access, in either synthetic grafts or native fistulas.
REFERENCES
- 1.Gaylord GM, Taber TE. Long-term hemodialysis access salvage: problems and challenges for nephrologists and interventional radiologists. J Vasc Interv Radiol. 1993;4:103–107. doi: 10.1016/s1051-0443(93)71830-8. [DOI] [PubMed] [Google Scholar]
- 2.Feldman HI, Held PJ, Hutchinson JT, et al. Hemodialysis vascular access morbidity in the United States. Kidney Int. 1993;43:1091–1096. doi: 10.1038/ki.1993.153. [DOI] [PubMed] [Google Scholar]
- 3.Gibbens DT, Triolo J, Yu T, et al. Contemporary treatment of thrombosed hemodialysis grafts. Tech Vasc Interv Radiol. 2001;4:122–126. doi: 10.1016/s1089-2516(01)90007-1. [DOI] [PubMed] [Google Scholar]
- 4.Moossavi S, Regan JD, Pierson ED, et al. Non-surgical salvage of thrombosed arterio-venous fistulae: a case series and review of the literature. Semin Dial. 2007;20:459–464. doi: 10.1111/j.1525-139X.2007.00356.x. [DOI] [PubMed] [Google Scholar]
- 5.Morgan R, Belli AM. Percutaneous thrombectomy: a review. Eur Radiol. 2002;12:205–217. doi: 10.1007/s003300101014. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz-Rode T, Bohndorf K, Gunther RW. New “mesh basket” for percutaneous removal of wall-adherent thrombi in dialysis shunts. Cardiovasc Intervent Radiol. 1993;16:7–10. doi: 10.1007/BF02603029. [DOI] [PubMed] [Google Scholar]
- 7.Beathard GA, Welch BR, Maidment HJ. Mechanical thrombolysis for the treatment of thrombosed hemodialysis access grafts. Radiology. 1996;200:711–716. doi: 10.1148/radiology.200.3.8756920. [DOI] [PubMed] [Google Scholar]
- 8.Wu CC, Lin MC, Pu SY, et al. Comparison of cutting balloon versus high-pressure balloon angioplasty for resistant venous stenoses of native hemodialysis fistulas. J Vasc Interv Radiol. 2008;19:877–883. doi: 10.1016/j.jvir.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Wu CC, Wen SC. Cutting balloon angioplasty for resistant venous stenoses of dialysis access: immediate and patency results. Catheter Cardiovasc Interv . 2008;71:250–254. doi: 10.1002/ccd.21402. [DOI] [PubMed] [Google Scholar]
- 10.Trerotola SO, Vesely TM, Lund GB, et al. Treatment of thrombosed hemodialysis access grafts: Arrow-Trerotola percutaneous thrombolytic device versus pulse-spray thrombolysis. Arrow-Trerotola Percutaneous Thrombolytic Device Clinical Trial. Radiology . 1998;206:403–414. doi: 10.1148/radiology.206.2.9457193. [DOI] [PubMed] [Google Scholar]
- 11.Sacks D, Marinelli DL, Martin LG, Spies JB. Reporting standards for clinical evaluation of new peripheral arterial revascularization devices. J Vasc Interv Radiol. 2003;14:S395–5404. doi: 10.1097/01.rvi.0000094613.61428.a9. [DOI] [PubMed] [Google Scholar]
- 12.Clinical practice guidelines for vascular access. Am J Kidney Dis . 2006;48:S176–S247. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Vesely TM, Hovsepian DM, Darcy MD, et al. Angioscopic observations after percutaneous thrombectomy of thrombosed hemodialysis grafts. J Vasc Interv Radiol . 2000;11:971–977. doi: 10.1016/s1051-0443(07)61324-4. [DOI] [PubMed] [Google Scholar]
- 14.Bech FR, Galt SW, Cronenwett JL. Increased platelet deposition on polytetrafluoroethylene grafts after balloon catheter thrombectomy. J Vasc Surg. 1990;11:804–810. doi: 10.1067/mva.1990.20069. [DOI] [PubMed] [Google Scholar]
- 15.Vesely TM, Williams D, Weiss M, et al. Comparison of the AngioJet Rheolytic Catheter to surgical thrombectomy for the treatment of thrombosed hemodialysis grafts. J Vasc Interv Radiol . 1999;10:1195–1205. doi: 10.1016/s1051-0443(99)70220-4. [DOI] [PubMed] [Google Scholar]
- 16.Heye S, Van Kerkhove F, Claes K, Maleux G. Pharmacomechanical thrombectomy with the Castaneda brush catheter in thrombosed hemodialysis grafts and native fistulas. J Vasc Interv Radiol. 2007;18:1383–1388. doi: 10.1016/j.jvir.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Turmel-Rodrigues L, Sapoval M, Pengloan J, et al. Manual thromboaspiration and dilation of thrombosed dialysis access: mid-term results of a simple concept. J Vasc Interv Radiol. 1997;8:813–824. doi: 10.1016/s1051-0443(97)70666-3. [DOI] [PubMed] [Google Scholar]
- 18.Littler P, Cullen N, Gould D, et al. AngioJet thrombectomy for occluded dialysis fistulae: outcome data. Cardiovasc Intervent Radiol. 2009;32:265–270. doi: 10.1007/s00270-008-9478-2. [DOI] [PubMed] [Google Scholar]
- 19.Wen SC, Pu SY, Tsai KC, et al. AngioJet thrombectomy to salvage thrombosed native dialysis fistulas. Acta Cardiol Sin . 2011;27:101–108. [Google Scholar]
- 20.Beathard GA, Litchfield T. Effectiveness and safety of dialysis vascular access procedures performed by interventional nephrologists. Kidney Int . 2004;66:1622–1632. doi: 10.1111/j.1523-1755.2004.00928.x. [DOI] [PubMed] [Google Scholar]
- 21.Swan TL, Smyth SH, Ruffenach SJ, et al. Pulmonary embolism following hemodialysis access thrombolysis/thrombectomy. J Vasc Interv Radiol. 1995;6:683–686. doi: 10.1016/s1051-0443(95)71164-2. [DOI] [PubMed] [Google Scholar]
- 22.Harp RJ, Stavropoulos SW, Wasserstein AG, Clark TW. Pulmonary hypertension among end-stage renal failure patients following hemodialysis access thrombectomy. Cardiovasc Intervent Radiol . 2005;28:17–22. doi: 10.1007/s00270-004-0223-1. [DOI] [PubMed] [Google Scholar]
- 23.McLennan G, Trerotola SO, Davidson D, et al. The effects of a mechanical thrombolytic device on normal canine vein valves. J Vasc Interv Radiol. 2001;12:89–94. doi: 10.1016/s1051-0443(07)61409-2. [DOI] [PubMed] [Google Scholar]
- 24.Lajvardi A, Trerotola SO, Strandberg JD, et al. Evaluation of venous injury caused by a percutaneous mechanical thrombolytic device. Cardiovasc Intervent Radiol. 1995;18:172–178. doi: 10.1007/BF00204145. [DOI] [PubMed] [Google Scholar]
- 25.Yang CC, Yang CW, Wen SC, Wu CC. Comparisons of clinical outcomes for thrombectomy devices with different mechanisms in hemodialysis arteriovenous fistulas. Catheter Cardiovasc Interv . 2012;80:1035–1041. doi: 10.1002/ccd.24408. [DOI] [PubMed] [Google Scholar]
- 26.Turmel-Rodrigues L, Raynaud A, Louail B, et al. Manual catheter-directed aspiration and other thrombectomy techniques for declotting native fistulas for hemodialysis. J Vasc Interv Radiol . 2001;12:1365–1371. doi: 10.1016/s1051-0443(07)61691-1. [DOI] [PubMed] [Google Scholar]
- 27.Shatsky JB, Berns JS, Clark TW, et al. Single-center experience with the Arrow-Trerotola percutaneous thrombectomy device in the management of thrombosed native dialysis fistulas. J Vasc Interv Radiol. 2005;16:1605–1611. doi: 10.1097/01.RVI.0000182157.48697.F5. [DOI] [PubMed] [Google Scholar]
- 28.Liang HL, Pan HB, Chung HM, et al. Restoration of thrombosed Brescia-Cimino dialysis fistulas by using percutaneous transluminal angioplasty. Radiology . 2002;223:339–244. doi: 10.1148/radiol.2232010821. [DOI] [PubMed] [Google Scholar]
- 29.Lai CC, Mar GY, Fang HC, et al. A novel intragraft pressure-guided technique versus angiography-guided technique in salvage of thrombosed hemodialysis graft: a randomized controlled trial. Acta Cardiol Sin. 2012;28:324–331. [Google Scholar]