Abstract
Aim:
The tuber of Amorphophallus paeoniifolius (Family-Araceae), commonly called suran or jimikand, has medicinal and food value. It is used in ethnomedicinal practices for correction of gastrointestinal disturbances such as constipation and hemorrhoids. The present study evaluated the effect of A. paeoniifolius tuber on gastrointestinal motor functions.
Materials and Methods:
The tuber was collected in December 2011, and its methanolic extract was standardized with the major phenolic compound, betulinic acid, by high-performance liquid chromatography. Rats were orally administered methanolic (APME) or aqueous (APAE) extract (250 and 500 mg/kg, each) of tuber for 7 days. Metoclopramide (MET) (3 mg/kg, orally) was used a reference prokinetic drug. The gastrointestinal parameters viz. number of feces, wet and dry weight and moisture content of feces, gastric emptying, and intestinal transit were evaluated. The isolated tissue preparations were used to check the effect of the extracts on fundus and intestinal contractility. The glucomannan and total phenolic and flavonoid contents were determined spectrophotometrically.
Results:
The pre-treatment of extracts significantly increased the number of feces, wet and dry weight of feces, moisture content, gastric emptying, and intestinal transit. Results were comparable to MET. Further, APME and APAE showed a contraction of fundus and ileum in isolated preparations. APME and APAE were also found to have fair amount of glucomannan, total phenolics, and flavonoids. The results indicate the gastrokinetic potential of the tuber extracts. This may be attributed to the presence of glucomannan and betulinic acid present in the extracts.
Conclusion:
In conclusion, the tuber of A. paeoniifolius exhibits gastrokinetic activity and substantiates its traditional use in gastrointestinal motor disturbances.
KEY WORDS: Betulinic acid, gastric emptying, glucomannan, intestinal transit, jimikand
INTRODUCTION
The disorders associated with bowel movements in gastrointestinal system include constipation, diarrhea, and functional bowel disorders. These diseases are not so severe or life-threatening, however, they badly affect the quality of life [1]. There is a high prevalence of some of the main gastrointestinal disorders and diseases worldwide. According to the survey data, the leading countries with a highest prevalence rate of functional dyspepsia, functional constipation, gastroesophageal reflux disease, and irritable bowel syndrome are Argentina (43.2%), Australia (6.3-10.3%), Argentina (11.9%), and Australia (8.9%), respectively [2]. Plant-based medicines are very popular for the treatment of gastrointestinal disturbances. The Ayurveda, the Indian system of medicine also advocates the use of many plant-based medicines for such disorders. In India, the plants/herbs with medicinal values constitute an integral part of the food to derive therapeutic benefits present in them in addition to source of nutrition [3].
Amorphophallus paeoniifolius (Dennst.) Nicolson (family - Araceae) or Elephant foot yam is a crop of South East Asian origin. In India, it is commonly known as suran or jimikand. The tuber of this plant has high medicinal value and consumed by many people as food. It is an important constituent of many Ayurvedic preparations [4]. The phytoconstituents present in the corms are quercetin, rutin, sitosterol, etc. [5]. A water-soluble polysaccharide containing galactose, glucose, 4-O-acyl-methyl galacturonate, and arabinose was isolated from the aqueous extract of tuber [6]. Glucomannan was also isolated from the tuber and characterized by spectroscopy [7]. High-performance thin layer chromatography analysis showed the presence of quercetin and gallic acid in the tuber [8]. Betulinic acid is a major phytochemical present in the methanolic extract of tuber [9].
The tuber is irritant, digestive, carminative, stomachic, and appetizer and has got remarkable effects on gastrointestinal system. It corrects various abnormalities viz. hemorrhoids, vomiting, anorexia, dyspepsia, flatulence, colic, constipation, hepatopathy, etc. [10]. It is used in ethnomedicinal practices for treatment of piles (hemorrhoids), abdominal pain, and constipation [11,12]. It is used for treatment of other conditions such as splenopathy, arthralgia, elephantiasis, tumors, inflammations, hemorrhages, cough, helminthiasis, bronchitis, asthma, amenorrhea, dysmenorrhea, seminal weakness, fatigue, anemia, and general debility. Pharmacologically, it has been demonstrated to exhibit analgesic activity [13], CNS depressant activity [14], anti-inflammatory activity [15], cytotoxic activity [16], antibacterial activity, and antifungal activity [17] in experimental studies.
Despite the myriad of actions of tuber of A. paeoniifolius in gastrointestinal system and its disturbances, there are no scientific studies to delineate its influence on the gastrointestinal functions. This study investigated the effect of tuber extract on motor and contractile gastrointestinal function in normal rats.
MATERIALS AND METHODS
Drug and Chemicals
Acetylcholine (Himedia Laboratories Pvt. Ltd. Mumbai), D-mannose, 3,5-DNS (di nitro salicylic acid) and Folin and ciocalteu’s reagent (Sisco Research Laboratories Pvt. Ltd., Mumbai), and tannic acid (Merck Chemicals, Mumbai) were procured from local market while quercetin was procured from Sigma-Aldrich, USA. All the reagents and chemical used were of highest purity grade.
Collection and Authentication of the Tuber
The tubers of A. paeoniifolius were collected from the local market of Gwalior in December 2011 and identified by Dr. N.K. Pandey, Taxonomist of the Institute. A voucher specimen No. 5-4/10-11/NRIASHRD/Tech/Survey/1611 was deposited in the herbarium of the Institute.
Preparation of Methanolic (APME) and Aqueous (APAE) Extract of A. paeoniifolius Tuber
The tubers were chopped into thin pieces, shade dried, and coarsely powdered. The powdered tuber was extracted with methanol in Soxhlet extractor. The marc was finally macerated with distilled water to obtain aqueous extract. The extracts were dried in a rotary evaporator and stored in a desiccator for further use. The methanolic extract of reddish brown semisolid consistency and aqueous extract of brown solid consistency were obtained with percent yield of 9.48% w/w and 6.16% w/w, respectively.
Preliminary Phytochemical Screening
Preliminary phytochemical screening was carried out to detect the presence and absence of various phytoconstituents such as carbohydrates, proteins, steroids, flavonoids, tannins, and other phenolic compounds, glycosides, and alkaloids in the extracts [18].
Quantitative Estimation of Phytoconstituents
The total glucomannan content was determined as described previously [19] and expressed as a gram of glucomannan per 100 gram of extract. The total phenolic content of the extracts was determined spectrometrically [20] and expressed as mg of tannic acid equivalents (TAE) per gram of extract while total flavonoid content was measured by aluminum chloride colorimetric assay [21] and expressed as mg of quercetin equivalent per gram of extract.
Standardization of APME by High-performance Liquid Chromatography (HPLC)
For the standardization, the betulinic acid, a chief constituent of tuber was estimated in the methanolic extract. The estimation of betulinic acid was carried out in APME by HPLC at Natural Remedies Pvt. Ltd., Bangalore, Karnataka, India. The mobile phase consisted of potassium dihydrogen orthophosphate buffer (0.136 g anhydrous KH2PO4 in 900 ml HPLC grade water and 0.5 mL of orthophosphoric acid added to mixture and volume was made up to 1000 ml with water) and acetonitrile in the proportion of 85:15. The column and detector used were phenomenex-luna C-18 (2) of size 250 × 4.60 mm and 5 µm internal diameter and photodiode array detector, respectively. The wavelength, flow rate, and injection volume were 205 nm, 1.5 ml/min, and 20 µl, respectively. Standard of Betulinic acid (Natural Remedy, India, percent purity ≥ 95%) (0.2 mg/ml) or APME (20 mg/ml) was prepared in HPLC grade methanol, and solutions were filtered through 0.2 µ membrane filter. The chromatograms were recorded, and the mean area (n = 3) and relative standard deviation were calculated. The amount of betulinic acid was calculated by the following formula:
Amount of betulinic acid = (area of the sample/area of the standard) × (weight of the standard [mg]/standard dilution [ml]) × (sample dilution [ml]/weight of the sample [mg]) × purity of the standard (%)
Animals
Healthy adult male Wistar rats (8-10 weeks age and 220-250 g weight) were used for the study. The animals were bred and maintained at Central Animal Facility of the Institute under standard experimental conditions of temperature (25 ± 1°C), relative humidity (50 ± 5%), and 12 h light:dark cycle. They were housed in polypropylene cages in a group of 2-3 animals/cage. They were fed standard rodent chow (Ashirwad brand, Chandigarh, India) and water ad libitum. Experiments were performed in accordance with the guidelines of Committee for the Purpose of Control and Supervision of Experimental Animals (CPCSEA), Ministry of Environment and Forest, New Delhi after seeking approval by the Institutional Animals Ethical Committee (IAEC) in the year 2013 (Proposal No. NRIASHRD-GWL/IAEC/2013/01).
Acute Toxicity Study
Acute oral toxicity study was carried out to determine the safe dose by acute toxic class method as per Organization for Economic Cooperation and Development (OECD) 423 guidelines [22]. The overnight fasted rats (n = 3) were orally administered APME and APAE in the limit test dose of 2000 mg/kg and observed continuously for behavioral, neurological, and autonomic profiles for 2 h and after a period of 24-h, 72 h, and thereafter up to 14 days for any lethality, moribund state, or death. The limit test was repeated in another group of rats (n = 3) for confirmation and toxic class of LD50 determination.
Grouping and Treatments
The animals (n = 6) were divided into 6 groups. APME and APAE were suspended in 1% tween 80 for the purpose of administration.
Group I: Control, received 1% Tween 80 as vehicle (5 ml/kg/day orally).
Group II-III: Fed orally with methanolic extract of A. paeoniifolius at the doses of 250 and 500 mg/kg.
Groups IV-V: Fed orally with aqueous extract of A. paeoniifolius at the doses of 250 and 500 mg/kg.
Group VI: Fed orally with metoclopramide (MET) at a dose of 3 mg/kg.
Evaluation of Gastrointestinal Motor Functions
The treatments were given for seven consecutive days and effect on gastrointestinal motor functions was assessed on the 8th day. The following parameters were assessed.
Feces and Stool Consistency
Each animal was kept individually in metabolic cage (Orchid Scientifics, Nasik, India) for four hours every day from 10 am to 2 pm. After 4 h, the number of feces and stool consistency were recorded. The feces were collected and weighed. The wet feces were dried in hot air oven at 105°C until constant weight was acquired. The drying temperature was kept constant in all feces. The drying time taken by feces to dry was different depending on the moisture content of the feces. The dry weight of feces was measured, and the moisture content of feces was then calculated by the following formula:
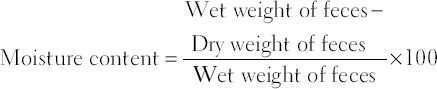
The average number of feces, wet and dry weight of feces, and moisture content of feces were calculated of each individual rat.
Gastric Emptying and Intestinal Transit
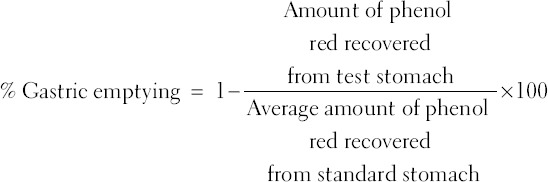
The gastric emptying was assessed in overnight fasted rats by measuring emptying of a non-nutrient solution as described previously [23]. Briefly, each rat received a 1.5 ml test meal consisting of 0.05% phenol red in 1.5% aqueous methylcellulose solution by intragastric route. After 30 min, rats were sacrificed by a high dose of ether. The abdomen was cut opened, and stomach was dissected out after careful ligation at the cardiac and pyloric ends and washed with normal saline. The stomach was cut into pieces and homogenized with 25 ml of 0.1 N NaOH. To this 5 ml homogenate, 0.5 ml of trichloroacetic acid (20% w/v) was added and centrifuged at 3000 rpm for 20 min. To one ml of supernatant, 4 ml of 0.5 N NaOH was added. The absorbance of the pink colored liquid was measured spectrophotometrically at 560 nm. Phenol red recovered from the stomach of rat sacrificed immediately after meal was considered as the average amount of phenol red from standard stomach. The percent gastric emptying was calculated as below.
The distance traveled by the phenol red meal in the intestine, from the pylorus to the cecum was measured and expressed as percent intestinal transit [24].
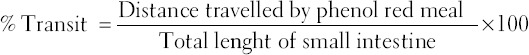
Fundus and Ileum Contractility [25]
Separate group of adult Wistar albino rats were fasted overnight with free access to water, and then they were sacrificed. The stomach was dissected out and placed in Krebs solution at 37°C. The gray fundal part was separated, cut longitudinally to strips and mounted in the organ bath as per standard procedure. Various concentrations of acetylcholine (1, 2, 4, 6, 8, 16, 32, and 64 µg) from the stock solution (10 µg/ml) or APME or APAE (1, 2, 4, 8, and 16 mg) from the stock solution (10 mg/ml) were injected into the tissue bath until maximum ceiling effect was observed. The concentration-response curves of acetylcholine, APME, and APAE were plotted.
From the same group of rats, the rat ileum preparation was mounted as per standard procedure. Various concentrations of acetylcholine (1, 2, 4, 6, 8, 16, 32, and 64 µg) from the stock solution (10 µg/ml) or APME and APAE (1, 2, 4, 8, and 16 mg) from the stock solution (10 mg/ml) were injected into the tissue bath containing tyrode solution until maximum response was observed. The concentration-response curves of acetylcholine, APME, and APAE were plotted.
Statistical Analysis
The data were analyzed with one-way ANOVA followed by Tukey’s multiple comparison post-hoc tests and two-way ANOVA followed by Bonferroni post-hoc tests. A statistical difference of P < 0.05 was considered significant in all cases.
RESULTS
Percentage Yield and Preliminary Phytochemical Screening
APME and APAE showed the presence of carbohydrates, proteins, alkaloids, flavonoids, sterols, phenolic compounds, and tannins while glycosides and saponins were found absent.
Quantitative Estimation of Phytoconstituents
The total flavonoid contents of APME and APAE were found to be 92.77 mg and 75.47 mg quercetin equivalents/g of extract, respectively, while the total phenolic contents of APME and APAE were found to be 73.1 mg and 141.5 mg TAE/g of extract, respectively. The glucomannan content of the APME and APAE was found to 1.13 and 9.043 g per 100 g of extract, respectively.
Estimation of Betulinic Acid
HPLC analysis revealed the presence of betulinic acid in APME. The peak of standard betulinic acid and APME solution are shown in Figure 1a and b, respectively. The estimated amount of betulinic acid was found to be 0.08 (% w/w) [Figure 1].
Figure 1.
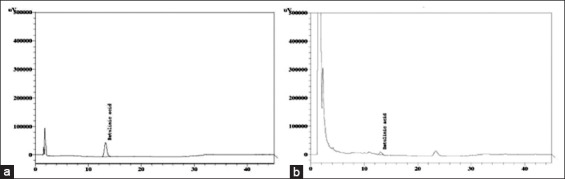
High-performance liquid chromatography (HPLC) fingerprint of administered methanolic, The HPLC fingerprint of (a) reference standard (retention time 13.077) and (b) methanolic extract of tuber (retention time = 13.045) at wavelength of 205 nm for detection of the marker compound, betulinic acid
Acute Toxicity Study of APME and APAE
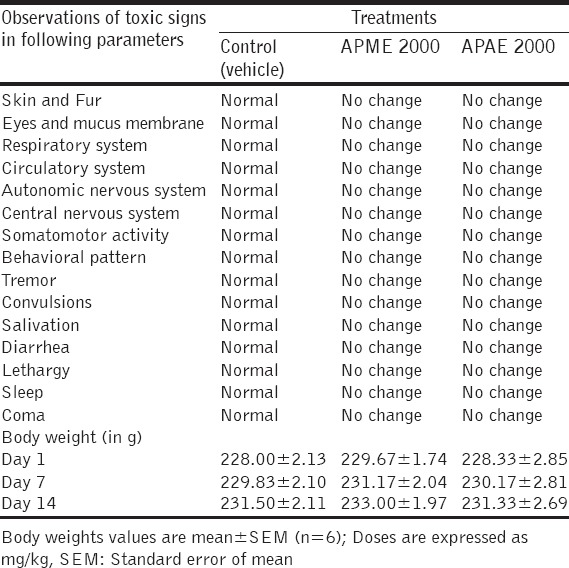
Acute toxicity studies revealed that both APME and APAE were safe up to a dose level of 2000 mg/kg of body weight (limit test) and LD50 observed was more than 2500 mg/kg. No lethality or any toxic reactions or moribund state were observed up to the end of the study period [Table 1]. Two-way ANOVA revealed that there were no significant changes observed in weekly body weights of rats treated with APME and APAE at 2000 mg/kg when compared to vehicle.
Table 1.
Acute toxicity of APME and APAE
Effects on Gastrointestinal Functions
Fecal parameters
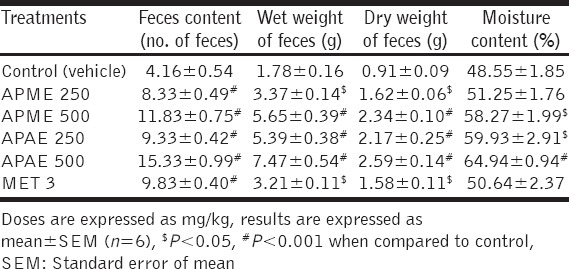
One-way ANOVA showed that APME and APAE have a significant influence on the number, wet and dry weight, and moisture content of feces. Post-hoc test indicated that APME or APAE at the dose of 250 and 500 mg/kg significantly increased the number (P < 0.001), wet weight (P < 0.05 and P < 0.001, wherever applicable), dry weight (P < 0.05 and P < 0.01, wherever applicable), and moisture content (P < 0.05 and P < 0.001, wherever applicable) of feces of rats as compared to control group [Table 2]. MET (3 mg/kg) also caused significant increase in the number (P < 0.001), wet weight (P < 0.05), dry weight (P < 0.05) of feces without significant change in moisture content.
Table 2.
Effects of APME and APAE on fecal parameters
Gastric Emptying and Intestinal Transit
One-way ANOVA showed that APME and APAE have a significant influence on gastric emptying and intestinal transit. Post-hoc test indicated that APME (250 and 500 mg/kg) or APAE at the dose of 500 mg/kg significantly increased the percent gastric emptying (P < 0.01-P < 0.001, respectively), whereas APME or APAE at both doses significantly (P < 0.05 - P < 0.001) increased the percent intestinal transit in rats as compared to control group [Figure 2]. MET (3 mg/kg) also showed significant increase in the gastric emptying (P < 0.001) and intestinal transit (P < 0.05). The effects of extracts were comparable to MET (P > 0.05).
Figure 2.
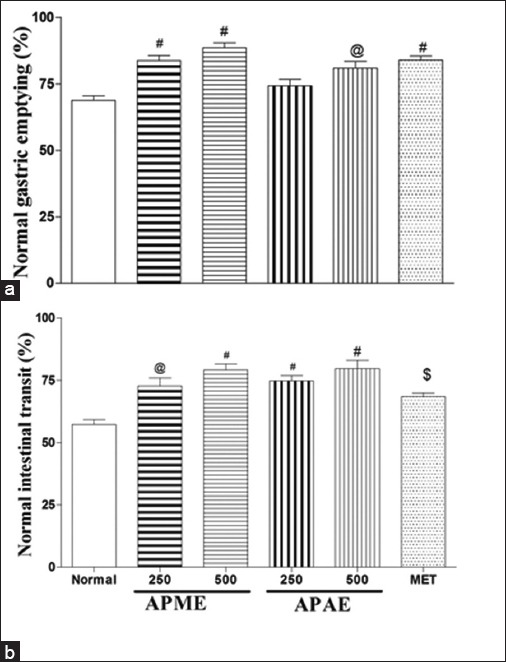
Effect of APME and APAE on Gastric emptying (a) and Intestinal transit (b). Doses are expressed as mg/kg, results are expressed as mean ± SEM (n=6), @P < 0.01 and *P < 0.001 when compared to control
Fundus and Ileum Contractility
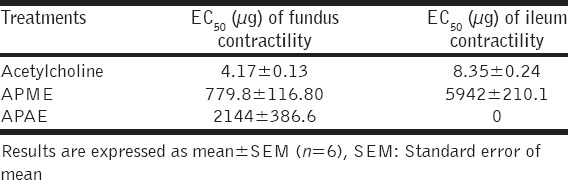
The concentration-response curve of acetylcholine or APME or APAE showed concentration-dependent increase in the contractile response on fundus and ileum. The EC50 (effective concentration for 50% response) were calculated and shown in Table 3. The EC50 of acetylcholine on fundus was 4.17 µg while that of APME and APAE were 779.8 and 2144 µg, respectively. Similarly, EC50 of acetylcholine and APME on ileum were 8.35 and 5942 µg, respectively.
Table 3.
Effect of APME and APAE on fundus and ileum contractility
DISCUSSION
Acute toxicity study of the APME and APAE revealed that there was no visible toxicity of any nature or moribund stage, and the drug was safe on oral administration. Based on cut-off LD50 value of >2500 mg/kg and previous studies [8,13], the dose range of 250 and 500 mg/kg (one tenth of the approximate LD50) was selected for drug administration. The increase in the number of feces as well as wet and dry weight of feces by APME or APAE treatment indicates increased bowel movement (peristalsis) and suggests that extracts have gastrokinetic effect [26]. The increased moisture content of feces due to extract treatment indicates the secretary action of extract in gastrointestinal tract, which further facilitates the enhanced peristalsis. The dietary fibers are known to affect gastrointestinal transit time and bulkiness of feces by increasing the water content and their bacterial degradation [27,28]. The tuber is known to contain nearly 70% of carbohydrates [29] and has high glucomannan water-soluble fiber content [7]. The phytochemical studies revealed the presence of fair amount of glucomannan in APAE and APME. Glucomannan has been reported to increase the feces volume and bulkiness of stool [30,31]. This supports and attributes the role of glucomannan in the observed increase in feces number, and weight and moisture content by APME or APAE contributing to prokinetic effect. Several dietary fibers such as Psyllium showed gastrokinetic action by increasing the transit, fecal wet and dry weight and moisture content [27,32], and strengthens the findings.
Further, APME and APAE both increased gastric emptying and intestinal transit of non-nutrient meal same as that of standard prokinetic drug-MET (P > 0.05). This further supports the gastrokinetic action of the extracts. MET exhibits prokinetic action due to its weak 5-HT3 antagonistic and 5-HT4 agonistic action [33]. Although there are no reports on the interaction of A. paeoniifolius with serotonergic system in gastrointestinal neurotransmission, it is possible that APME and APAE may have influence on serotonergic system in exhibiting gastrokinetic effect.
The increased peristalsis, gastric emptying, and intestinal transit suggest that the extracts have an influence on gastric motility or contractile function of the stomach/intestine. In affirmation, APME or APAE showed enhancement in gastric motility as observed by increased fundus and ileum contractility [Table 3]. However, the effect of the extracts was very less potent as indicated by higher EC50 values of APME and APAE compared to acetylcholine. It is very difficult to ascertain the role of exact constituent in influencing gastric motility. Previously, ferulic acid, a phenolic acid, showed stimulatory effect on the production of prostaglandins and caused increased gastric motility [26]. The HPLC analysis of APME also revealed the presence of 0.08% betulinic acid, a phenolic acid as chief constituent, in concordance to literature [9]. Though there are no direct evidences of gastrointestinal actions of betulinic acid from A. paeoniifolius, Bejar et al. [34] has demonstrated that betulinic acid isolated from Byrsonima crassifolia exhibited spasmogenic effect on rat fundus preparation. Thus, motility enhancing the effect of the extracts may be attributed to the presence of betulinic acid possibly through spasmogenic effect.
CONCLUSION
In conclusion, the tuber of A. paeoniifolius exhibited gastrokinetic action and validates its traditional use in correction of gastrointestinal disturbances. The gastrokinetic effect implicates the therapeutic potential of the plant in the correction of constipation, gastro paresis, and other functional bowel disorders.
ACKNOWLEDGMENTS
The authors acknowledge Natural Remedies, Bangalore, India, to carry out the HPLC analysis in the research work. The authors are thankful to the Director General, Central Council for Research in Ayurvedic Sciences, New Delhi for providing all the necessary facilities.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Saito T, Mizutani F, Iwanaga Y, Morikawa K, Kato H. Laxative and anti-diarrheal activity of polycarbophil in mice and rats. Jpn J Pharmacol. 2002;89:133–41. doi: 10.1254/jjp.89.133. [DOI] [PubMed] [Google Scholar]
- 2.World Gastroenterology Organization. Map of Digestive Disorders and Diseases. Milwaukee WI: 2008. [Last accessed on 2015 October 17]. Available from: http://www.worldgastroenterology.org/userfiles/file/wdhd-2008-map-of-digestive-disorders.pdf . [Google Scholar]
- 3.Mitra SK, Rangesh PR. Constipation (Vibandh) In: Mishra LC, editor. Scientific basis for Ayurvedic Therapies. 1st ed. Boca Raton, New York: CRC Press; 2004. pp. 323–38. [Google Scholar]
- 4.Anonymous. The Ayurvedic Formulary of India. 1st English ed. New Delhi: Department of ISM and H, Ministry of Health and Family Welfare, Government of India, (Part-II); 2000. pp. 205–7. [Google Scholar]
- 5.Sharstry RA, Biradar SM, Mahadevan KM, Habbu PV. Isolation and characterization of secondary metabolite from Amorphophallus paeoniifolius for hepatoprotective activity. Res J Pharm Biol Chem Sci. 2010;1:429–37. [Google Scholar]
- 6.Das D, Mondal S, Roy SK, Maiti D, Bhunia B, Maiti TK, et al. Isolation and characterization of a heteropolysaccharide from the corm of Amorphophallus campanulatus. Carbohydr Res. 2009;344:2581–5. doi: 10.1016/j.carres.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen TA, Do TT, Nguyen TD, Pham LD, Nguyen VD. Characterization of polysaccharide from Amorphophallus paeoniifolius in Vietnam. J Chem. 2009;47:155–9. [Google Scholar]
- 8.Nataraj HN, Murthy RL, Setty R. Evaluation of gastroprotective ability of Amorphophallus paeoniifolius corms against indomethacin induced gastric ulcers. RGUHS J Pharm Sci. 2012;2:67–73. [Google Scholar]
- 9.Tandan N, Sharma P. Quality Standards of Indian Medicinal Plants. New Delhi: Indian Council of Medical Research; 2013. pp. 39–47. [Google Scholar]
- 10.Nair RV. Indian Medicinal Plants. 3rd ed. Madras: Orient Longman; 1993. pp. 118–122. [Google Scholar]
- 11.Rahman AH, Nitu SK, Ferdows Z, Islam AK. Medico-botany on herbaceous plants of Rajshahi, Banglabesh. Am J Life Sci. 2013;1:136–44. [Google Scholar]
- 12.Devi Prasad AG, Shyma TB, Raghavendra MP. Plants used by the tribes for the treatment of digestive system disorders in Wayanad district, Kerala. J App Pharm Sci. 2013;3:171–5. [Google Scholar]
- 13.Shilpi JA, Ray PK, Sarder MM, Uddin SJ. Analgesic activity of Amorphophallus campanulatus tuber. Fitoterapia. 2005;76:367–9. doi: 10.1016/j.fitote.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Das SS, Sen M, Dey YN, De S, Ghosh AK. Effects of petroleum ether extract of Amorphophallus paeoniifolius tuber on central nervous system in mice. Indian J Pharm Sci. 2009;71:651–5. doi: 10.4103/0250-474X.59547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De S, Dey YN, Ghosh AK. Anti-inflammatory activity of methanolic extract of Amorphophallus paeoniifolius and its possible mechanism. Int J Pharm Biol Sci. 2010;1:1–8. [Google Scholar]
- 16.Angayarkanni J, Ramkumar KM, Poornima T, Priyadarshini U. Cytotoxic activity of Amorphophallus paeoniifolius tuber extracts in vitro. Am Eur J Agric Environ Sci. 2007;2:395–8. [Google Scholar]
- 17.Khan A, Rahman M, Islam MS. Antibacterial, antifungal and cytotoxic activities of amblyone isolated from Amorphophallus campanulatus. Indian J Pharmacol. 2008;40:41–4. doi: 10.4103/0253-7613.40489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khandelwal KR. Practical Pharmacognosy. 15th ed. Pune: Nirali Prakashan; 2006. pp. 149–56. [Google Scholar]
- 19.Chua M, Chana K, Hocking TJ, Williams PA, Perry CJ, Baldwin TC. Methodologies for the extraction and analysis of konjac glucomannan from corms of Amorphophallus konjac K. Koch. Carbohydr Polym. 2012;87:2202–10. [Google Scholar]
- 20.Singleton V, Orthofer R, Lamuela-Ravento’s R. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In: Packer L, editor. Oxidants and Antioxidants, Part A. Methods in Enzymology. New York: Academic Press; 1999. pp. 152–78. [Google Scholar]
- 21.Marinova D, Ribarova F, Atanasova M. Total phenolics and flavonoids in Bulgarian fruits and vegetables. J Univ Chem Tech Metall. 2005;40:255–60. [Google Scholar]
- 22.Organization for Economic Cooperation and Development (OECD) 2001 Guideline for Testing of Chemicals. Revised Draft Guideline 423. Document on Acute Oral Toxicity and Acute Toxicity Class Method. Paris: [Last accessed on 2013 Jul 30]. Available from: http://www.oecd.org . [Google Scholar]
- 23.Sharma SS, Gupta YK. Effect of antioxidants on cisplatin induced delay in gastric emptying in rats. Environ Toxicol Pharmacol. 1997;3:41–6. doi: 10.1016/s1382-6689(96)00137-8. [DOI] [PubMed] [Google Scholar]
- 24.Suchitra AD, Dkhar SA, Shewade DG, Shashindran CH. Relative efficacy of some prokinetic drugs in morphine-induced gastrointestinal transit delay in mice. World J Gastroenterol. 2003;9:779–83. doi: 10.3748/wjg.v9.i4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulkarani SK. Practical Pharmacology and Clinical Pharmacy. 1st ed. New Delhi: Vallabh Publications; 2008. pp. 115–6. [Google Scholar]
- 26.Badary OA, Awad AS, Sherief MA, Hamada FM. In vitro and in vivo effects of ferulic acid on gastrointestinal motility: Inhibition of cisplatin-induced delay in gastric emptying in rats. World J Gastroenterol. 2006;12:5363–7. doi: 10.3748/wjg.v12.i33.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens J, VanSoest PJ, Robertson JB, Levitsky DA. Comparison of the effects of psyllium and wheat bran on gastrointestinal transit time and stool characteristics. J Am Diet Assoc. 1988;88:323–6. [PubMed] [Google Scholar]
- 28.Cummings JH, Branch W, Jenkins DJ, Southgate DA, Houston H, James WP. Colonic response to dietary fibre from carrot, cabbage, apple, bran. Lancet. 1978;1:5–9. doi: 10.1016/s0140-6736(78)90357-4. [DOI] [PubMed] [Google Scholar]
- 29.Srivastava S, Verma D, Srivastava A, Tiwari SS, Dixit B. Phytochemical and nutritional evaluation of Amorphophallus campanulatus (Roxb.) Blume Corm. J Nutr Food Sci. 2014;4:1–6. [Google Scholar]
- 30.Tungland BC, Meyer D. Nondigestible oligo- and polysaccharides (dietary fiber): Their physiology and role in human health and food. Compr Rev Food Sci Food Saf. 2002;1:90–109. doi: 10.1111/j.1541-4337.2002.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 31.Chen HL, Cheng HC, Liu YJ, Liu SY, Wu WT. Konjac acts as a natural laxative by increasing stool bulk and improving colonic ecology in healthy adults. Nutrition. 2006;22:1112–9. doi: 10.1016/j.nut.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Hillemeier C. An overview of the effects of dietary fiber on gastrointestinal transit. Pediatrics. 1995;96:997–9. [PubMed] [Google Scholar]
- 33.Mahesh R, Perumal RV, Pandi PV. Cancer chemotherapy-induced nausea and vomiting: Role of mediators, development of drugs and treatment methods. Pharmazie. 2005;60:83–96. [PubMed] [Google Scholar]
- 34.Bejar E, Amarquaye A, Che CT, Malone MH, Fong HH. Constituents of Byrsonima crassifolia and their spasmogenic activity. Int J Pharmacogn. 1995;33:25–32. [Google Scholar]


