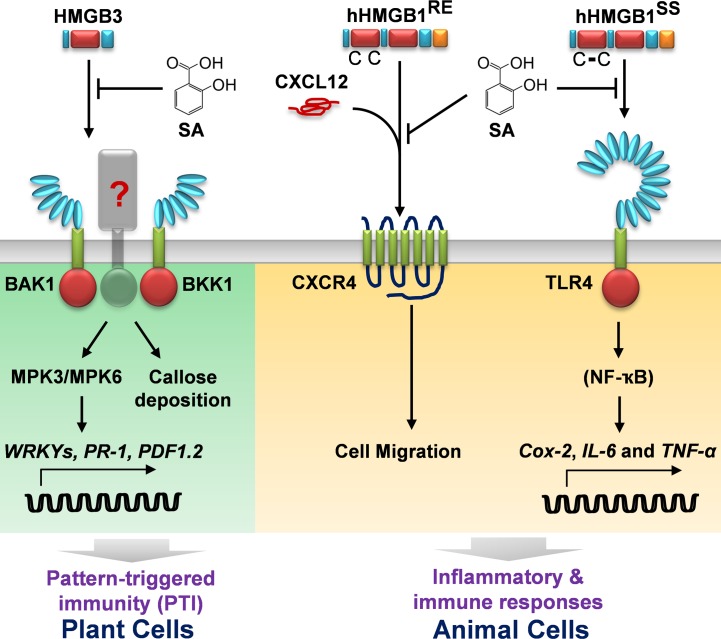
Fig 7. Schematic of the mechanisms through which SA inhibits the DAMP activities of HMGBs in plant and animal cells.
In plant cells, extracellular HMGB3 is recognized by a yet to be identified receptor complexed with the regulatory LRR RLKs BAK1 and/or BKK1. Recognition induces a series of pattern-triggered immune (PTI) responses, including MAPK activation (MPK3 and MPK6), defense-related gene expression (WRKYs, PR-1, and PDF1.2), and callose deposition. SA binding to extracellular HMGB3 inhibits its DAMP activity. In animal cells, extracellular HMGB1 is recognized by multiple cell surface receptors, including C-X-C chemokine receptor 4 (CXCR4) and toll-like receptor 4 (TLR4), depending on its redox state. SA inhibits chemo-attractant activity of reduced HMGB1 (hHMGB1RE), which is recognized by the cell surface receptor CXCR4 after heterocomplex formation of hHMGB1RE with C-X-C motif-containing chemokine 12 (CXCL12) [24,42]. SA also inhibits activation of Cox-2 and pro-inflammatory cytokine genes (IL-6 and TNF-α) by the disulfide-bonded form of HMGB1 (hHMGB1SS), which is mediated by heterocomplex formation of hHMGB1SS with myeloid differentiation factor 2 (MD-2), followed by signal transduction through the cell surface receptor TLR4 and transcription factor NF-κB [23,24].