Figure 1.
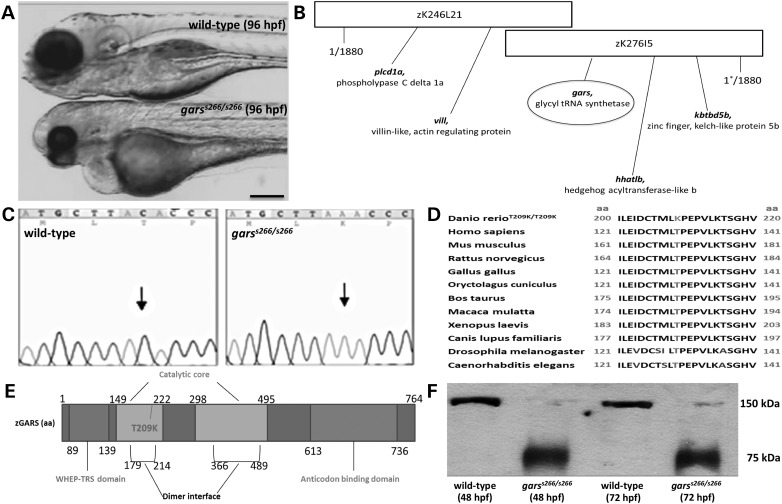
Identification and cloning of a novel gars allele. (A) Bright-field images of wild-type (A, upper), garss266/s266 mutant (A, lower) at 96 hpf. garss266/s266 embryos are delayed in development. They exhibit smaller head, eyes, body axis and a pericardial edema (B) The mutation was mapped on linkage group 24 (BAC clone DKEY-276I5) using 1880 mutant embryos. (C) s266 embryos carry a C->A transversion, leading to a substitution within the gars gene that changes a threonine to a lysine at residue 209. The gars sequence is shown at the region of the mutation from a wild-type (left panel) and a homozygous mutant (right panel). (D) The amino acid change is in a highly conserved region of GARS protein throughout species (conserved amino acids are in black; the altered lysine is highlighted). (E) gars is a 17-exon gene encoding 764-amino acid protein with two catalytic domains. The T209 mutant corresponds to the T130K in the human protein and is inside the first catalytic domain and in the dimer interface domain. (F) Using a specific human anti-GARS antibody to protein extracts from garss266/s266 and wild-type embryos at 48 and 72 hpf on a native western blot, we showed that T130K interferes with the dimerization of the protein. Scale bar: 100 μm.