Scheme 7. Scope of the Ru-Catalyzed Arylation of (Hetero)Aromatics 1c–z, 4–7 with Bromoarenes 2a–b.
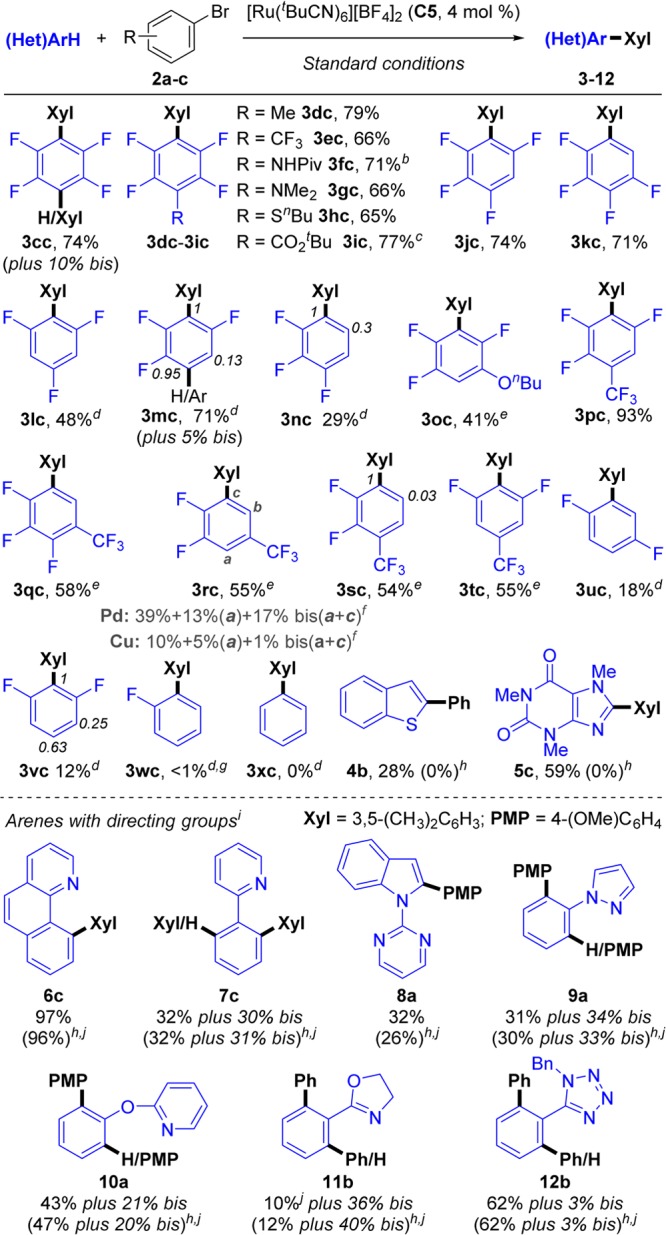
Standard conditions: bromoarene 2 (0.5 mmol), (fluoro)arene 1 (3 equiv), (NMe4)(4-FC6H4CO2) (0.35 equiv), (NMe4)OPiv (0.4 equiv), (NMe4)(OC(CF3)3) (2.5 equiv), C5 (4 mol %), and t-BuCN (3 equiv) stirred at 115 °C under N2 in a closed vessel for 16 h. Yields are of pure, isolated products.
t-BuCN (6 equiv).
Reaction time was 2 h.
1 (10 equiv).
1 (5 equiv).
See Figures 7 and S8 and Table S12.
Detected in traces by 19F NMR.
Yields in brackets are from reactions carried out without (NMe4)(4-FC6H4CO2).
(Hetero)arene (0.5 mmol) and bromoarene 2 (1 equiv).
Yield determined by 1H NMR analysis.
