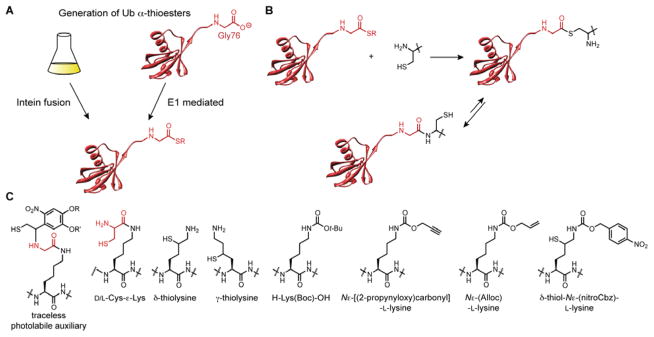
Figure 5.
Components of EPL. (A) Two different methods are available for generating recombinant Ub α-thioesters. The first method is based on the production of Ub-intein fusion proteins as thiolysis of the fusion protein leads to the desired α-thioester. The second method exploits the reactivity of E1 Ub-activating enzymes. This reaction proceeds through an E1-S-Ub acyl enzyme intermediate that is captured by a small molecule bearing a free thiol to afford Ub(1–76) α-thioester. (B) The mechanism of EPL with 1,2-aminothiols. The reaction commences with a transthioesterification followed by a rapid S-to-N acyl shift to afford a native amide bond. (C) Structures of various non-proteinogenic amino acids used to construct isopeptide linkages with Ub α-thioesters. Both H-Lys(Boc)-OH and Nε-[(2-propynyloxy)carbonyl]-L-lysine have not been used to forge an isopeptide linkage with Ub α-thioester; rather, other Ub C-terminal variants are required.102,104