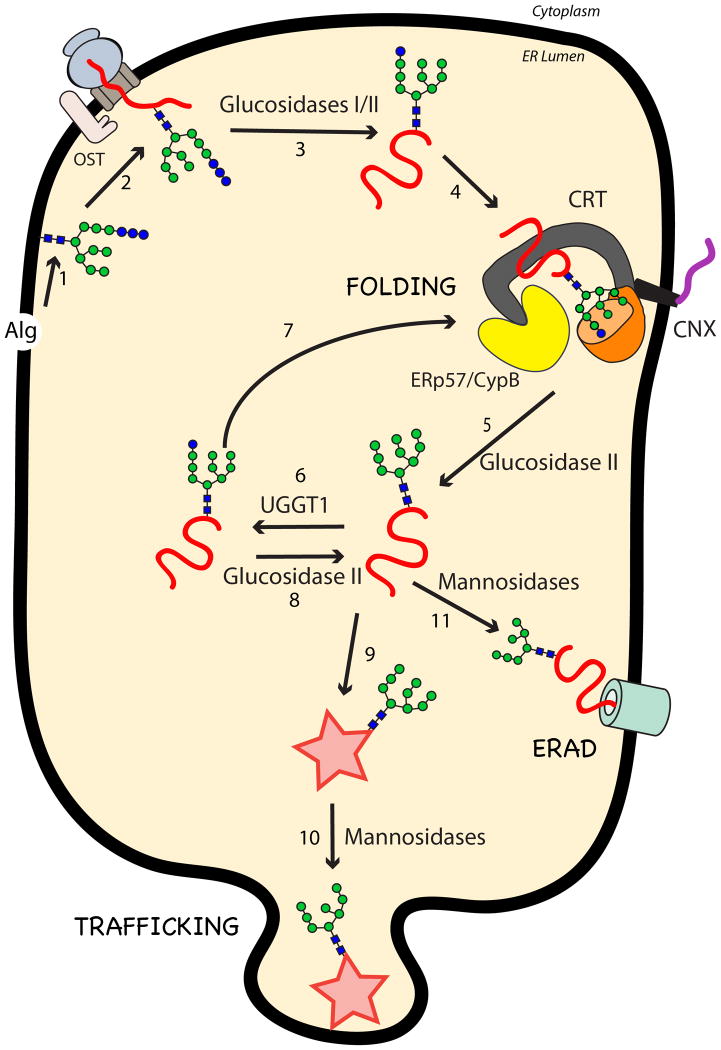
Figure 3. Processing of N-linked glycans and folding pathway of secretory glycoproteins in the Endoplasmic Reticulum.
N-linked glycans, assembled by Alg gene protein products (1), are transferred via the OST complex to proteins translocated into the ER lumen (2). Glucosidases I and II trim the first two glucoses (blue circles) from the glycan (3), generating a monoglucosylated glycoform, which is a substrate for calnexin (CNX) and calreticulin (CRT) (4). Trimming of the remaining glucose by glucosidase II releases the substrate from CNX/CRT (5). Glycoproteins that do not reach their native folded state can be reglucosylated by UGGT1 (6), redirecting them to CNX/CRT binding (7) or trimming by glucosidase II (8). Glycoproteins that have reached their native state (star) (9) are trimmed by mannosidases and directed for trafficking out of the ER (10). Proteins that are terminally misfolded are trimmed by mannosidases and directed for ERAD (11).