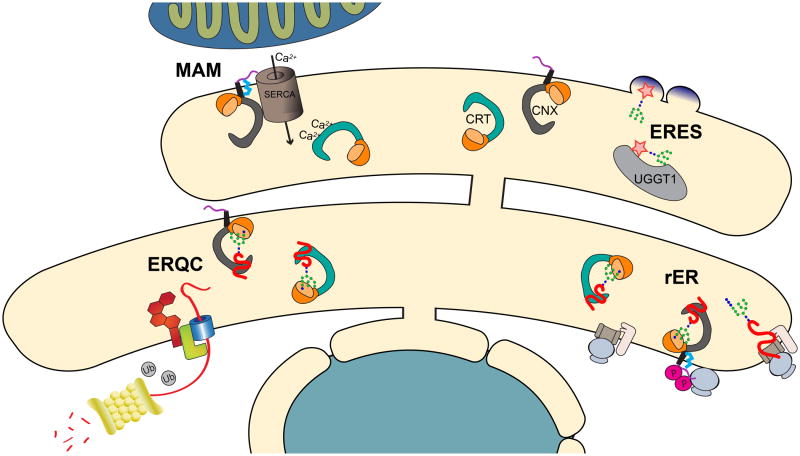
Figure 4. Cellular localization of lectin chaperone network.
In the rough ER (rER), which is characterized by the presence of ribosomes on the cytoplasmic side of the membrane and translocation machinery, glucosidase I and II sequentially trim glucoses from newly added N-linked glycans generating monoglucosylated glycoproteins. Calnexin and calreticulin associate with actively translating and translocating proteins. As proteins continue to fold, they migrate to the smooth portion of the ER where they are interrogated by UGGT1 in the peripheral ER at ER exit sites (ERES). If proteins are deemed terminally misfolded, they are targeted for ERAD. Calnexin, calreticulin and misfolded proteins accumulate in the ER quality control compartment (ERQC). The ER contacts the mitochondria at the mitochondria-associated membrane (MAM). Calnexin in particular is shuttled between the rER and the MAM by post-translational modifications based on the current needs of the cell. Calnexin is differentially phosphorylated (P –in yellow) on several residues within its C-terminal cytoplasmic tail, associating it with translating ribosomes and enriching it in the rER. Additionally, calnexin has been shown to be palmitoylated (in blue).