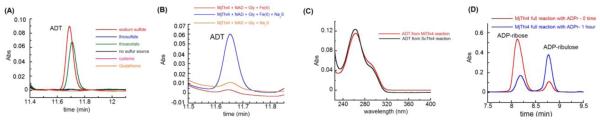
Figure 1.
(A) HPLC chromatogram showing formation of ADT 10 in the presence of different sulfur sources. Assays were carried out in the presence of 20 μM MjThi4, 500 μM ADP-ribose (ADPr) 2, 1 mM glycine, 400 μM ferrous ammonium sulfate (FeAS) and 0.8-1 mM of each of the sulfur compounds. Assays were quenched with 8 M GuHCl after 1 hour anaerobic incubation at 85 °C. (B) HPLC chromatogram showing conversion of NAD into ADT in the presence 100 μM MjThi4, 300 μM NAD, 1 mM glycine, 400 μM FeAS and 800 μM Na2S at 25 °C. (C) Overlaid UV-Vis spectra of the reaction product shown in panel A and an authentic sample of ADT prepared using ScTHI4. (D) Production of ADP-ribulose (ADPrl) 3 from ADPr 2 by MjThi4. Conditions were as follows; 500 μM MjThi4, 200 μM ADPr, 1 mM glycine, 1 mM FeAS and 800 μM Na2S inside a glovebox at 25 °C.