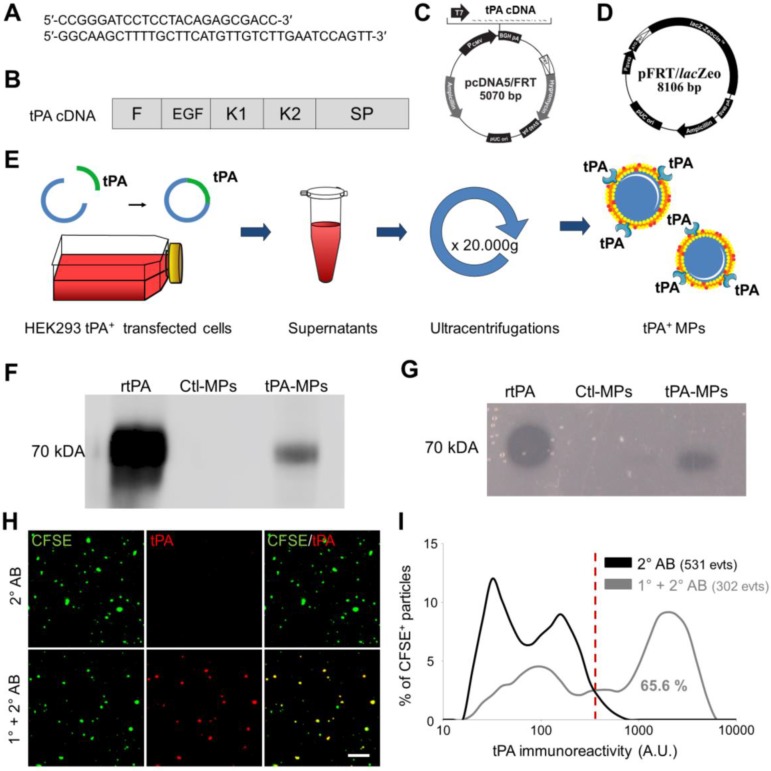
Figure 5.
Generation, detection and characterization of engineered cell-derived fibrinolytic MPs by LSCM. (A) Primers sequence (Forward and Reverse) that allowed whole human tPA cDNA sequence amplification. (B) Structure of human cDNA tPA sequence, including all tPA domains. (C) tPA plasmid. (D) Plasmid allowing stable integration of the human tPA in the cell genome. (E) Schematic representation of the method that allows tPA-MPs production from HEK293 cell cultures through stable transfection with plasmids containing tPA sequence with subsequent purification of released MPs by sequential ultracentrifugations. (F) Representative tPA immunoblot after migration of the proteins of MPs purified from tPA-expressing (tPA-MPs) or control non-transfected HEK-293 (Ctl-MPs). (G) Same as in (F) but representing a fibrin-agar zymography, demonstrating that the tPA in tPA-MPs is proteolytically active. (H) Representative images of tPA-MPs immunofluorescence with primary and secondary antibodies (1°+2° AB, red). (I) Quantification of tPA immunoreactivity in MPs incubated without (black) or with (grey) tPA primary antibody. Red vertical bar indicates baseline value (mean + 2*standard deviation of control condition) (n=3). tPA domains= F: finger, EGF: Epithelial Growth Factor, K: Kringle, SP: Serine Protease.