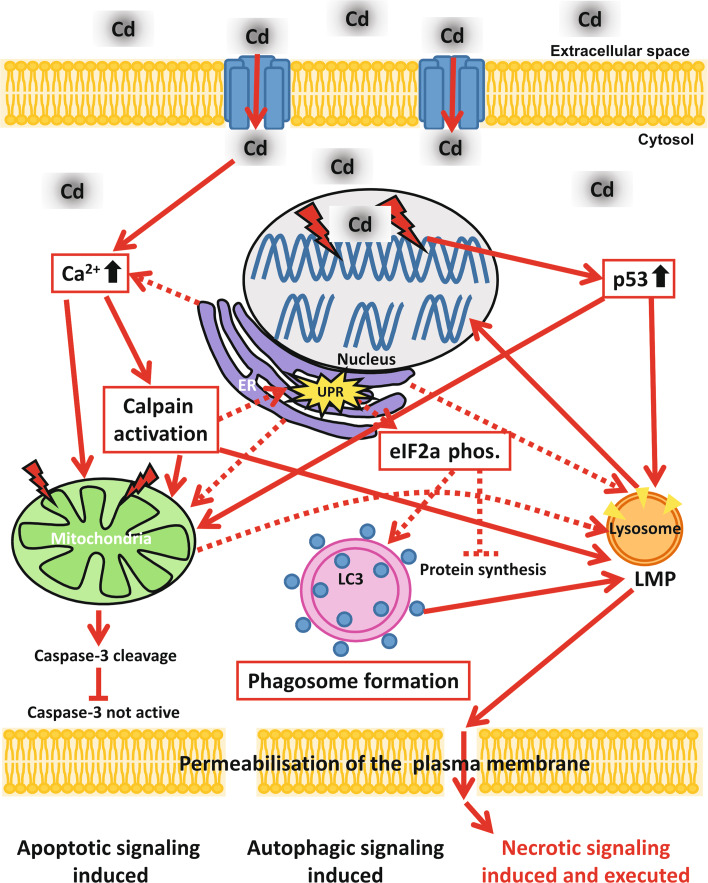
Fig. 8.
Schematic representation of Cd-induced signalling pathways in endothelial cells. After uptake of Cd in endothelial cells [possibly through divalent metal transporter 1 (DMT-1)] the cytosolic Ca2+ concentration rises. Besides being taken up from the extracellular space, Ca2+ could also originate from the ER. Redirection of the signal to the mitochondria could take place directly by Ca2+ or also indirectly by the activation of calpains. Forwarding of the apoptotic signalling from the mitochondria occurs via the cleavage of caspase-3, but the apoptotic signal is halted at this point as Cd treatment is known to simultaneously inhibit caspase-3 activity. Alongside affecting the mitochondria, Cd treatment impairs also the ER function and induces ER stress (shown by UPR). Cd-induced ER stress may stop protein synthesis via eIF2 alpha phosphorylation and also induce autophagic signalling, potentially as a survival mechanism. An additional target of Cd-induced toxicity in endothelial cells is cellular DNA. Cd-induced DNA damage results in upregulation of the DNA damage response protein p53, which in turn is shown to influence mitochondrial membrane potential. The central organelle in Cd-induced toxicity is the lysosome, as Cd-induced death signals from the mitochondria, the ER and p53 are finally “merging” at the lysosome, inducing LMP. LMP induction is in consequence responsible for complete DNA degradation and also for plasma membrane permeabilization and therefore ultimately for the execution of necrosis in Cd-treated endothelial cells although apoptotic as well as autophagy signals are triggered as well