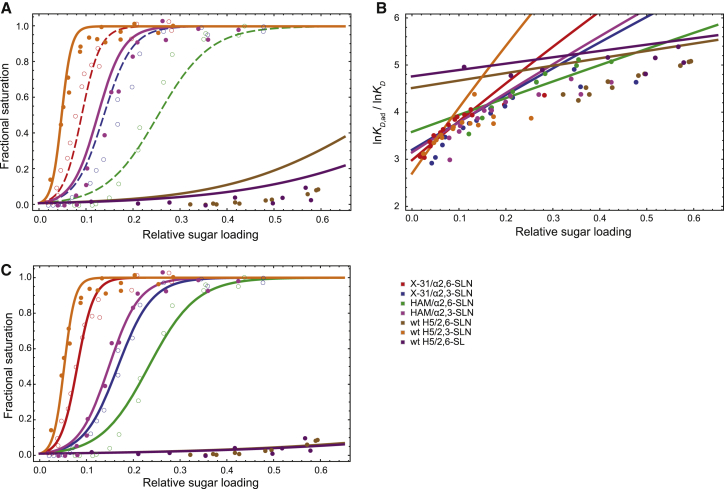
Figure 3.
The agreement between our model and experimental measurements of adhesion. (A) Isotherms of adhesion between influenza virus and biosensors loaded with SA. (Lines) Isotherms predicted by our model; (circles) experimental results. Different colors correspond to different combinations of viral strains and α2,3-SA or α2,6-SA. (Dashed lines and open circles) The three isotherms used for fitting the model parameters; (solid lines) predictions of isotherms for which experimental measurements are available. (B) Multiplicity of adhesion, m = lnKD,ad/lnKD, as a function of the relative sugar loading for different HA-SA pairs. The variation of m across different relative sugar-loading values is reasonably well captured by our model. (C) Computed and measured isotherms of adhesion, with KD values refitted to each individual isotherm, using the parameter values of K0 and Veff in Table 1. The fractional saturation predicted by our model is in good agreement with the experimental measurements, with fitted KD values close to those measured by MST. X-31, HAM, and wild-type (wt) H5 are different influenza strains, each with a different HA sequence and thus different binding affinities for sialic acids (19) (Table 2). α2,3-SLN (α2,3-linked sialyl lactosamine), α2,6-SLN (α2,6-linked sialyl lactosamine), and α2,6-SL (α2,6-linked sialyl lactose) are different terminal sialic acid moieties.