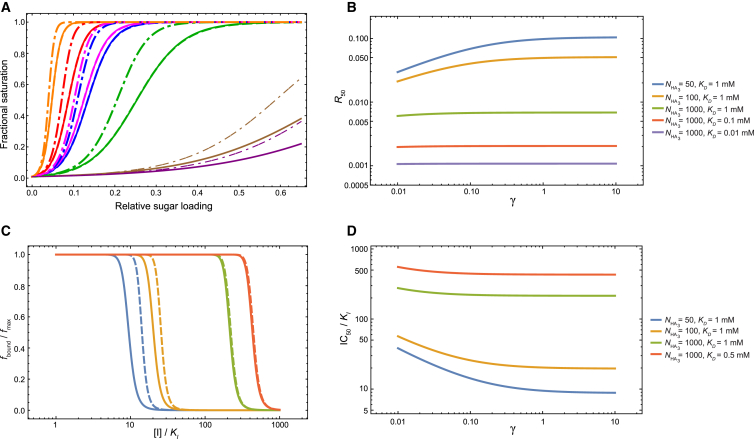
Figure 7.
The effect of binding cooperativity within the same HA trimer on the isotherms and the inhibition of adhesion. (A) Adhesion isotherms for the viral strains considered in this work. (Solid lines) Model without cooperativity (i.e., γ = 1), with the fit parameters in Table 1. (Dashed lines) Model refit with cooperativity, which yielded a value of γ with large statistical uncertainty. The parameters from the refitting are Veff = 2.6 × 103 mM−1, K0 = 1.2 × 10−5, and γ = 72. (Dashed lines are barely visible because they are almost on top of the solid lines, which indicates that the variations of the model that do and do not consider binding cooperativity produce very similar fits to the experiment.) (Dash-and-dot lines) Isotherms generated with the parameters in Table 1, but with γ = 0.1. (B) The dependence of R50 on the cooperativity parameter γ. (C) The effect of cooperativity on the inhibition of adhesion. (Solid lines) Inhibition of adhesion if there is no cooperativity (i.e., γ = 1); (dashed lines) inhibition of adhesion if the binding affinities of the second and third SA molecules are 10-fold stronger than that of the first SA molecule to the same HA trimer (γ = 0.1). (D) The dependence of IC50 values ([I]/KI at which the fraction of bound cells is half that in absence of the inhibitor) on cooperativity. The values of NSA, Veff, and K0 in Table 1 are used for (B)–(D). In (C), the cell concentration is as estimated in the text ([cell] = 4 × 10−10 mM). (B–D) Effect of cooperativity diminishes with the number of HA trimers.