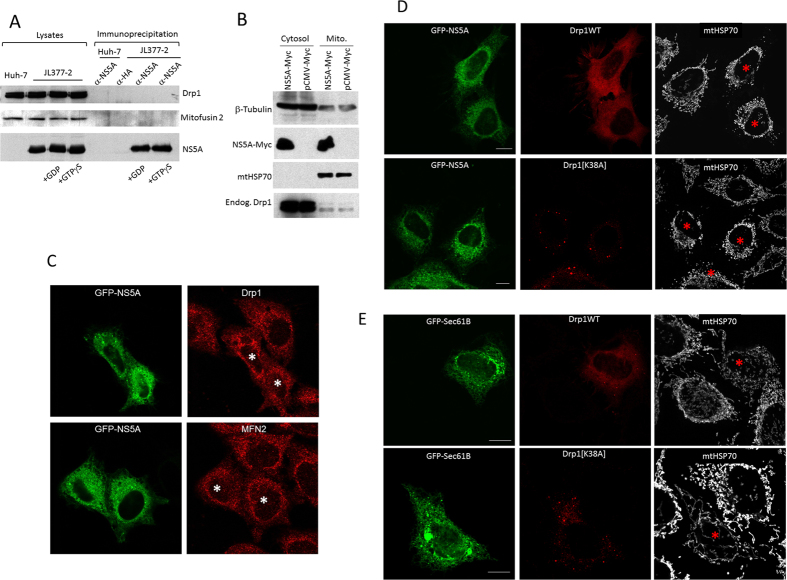
Figure 4. NS5A-induced mitochondrial fragmentation is independent of Drp1.
(A) NS5A does not interact with Drp1. Huh-7 or JL377-2 cell lysates were subjected to immunoprecipitation by α-NS5A antibody in the presence of GDP or GTPγS. A monoclonal antibody against HA was used as a control. In all conditions, neither endogenous Drp1 nor mitofusin 2 was co-precipitated with NS5A. (B) NS5A does not increase the mitochondrial association of Drp1. HEK293 cells overexpressed with Myc-NS5A or control Myc-empty vector and the extent of Drp1 on mitochondria-enriched membranes were determined by immunoblotting. (C) Overexpression of GFP-NS5A does not change the subcellular localization of endogenous Drp1 and mitofusin 2 (MFN2) by immunofluorescence. GFP-NS5A expressing cells are marked with asterisks. (D) Wildtype Drp1 or Drp1[K38A] dominant negative mutant cDNA sequences (red) were cotransfected with GFP-NS5A (green) into HEK293 cells. The mitochondria were visualized by staining the cells with mtHSP70 at 633 nm wavelength excitation laser (white). (E) Drp1 or Drp1[K38A] dominant negative mutant cDNA sequences (red) were cotransfected with control GFP-Sec61B (green) into HEK293 cells. The mitochondria were visualized by staining the cells with mtHSP70 at 633 nm wavelength excitation laser (white). Red asterisks indicate transfected cells. Scale Bar = 10 μm.