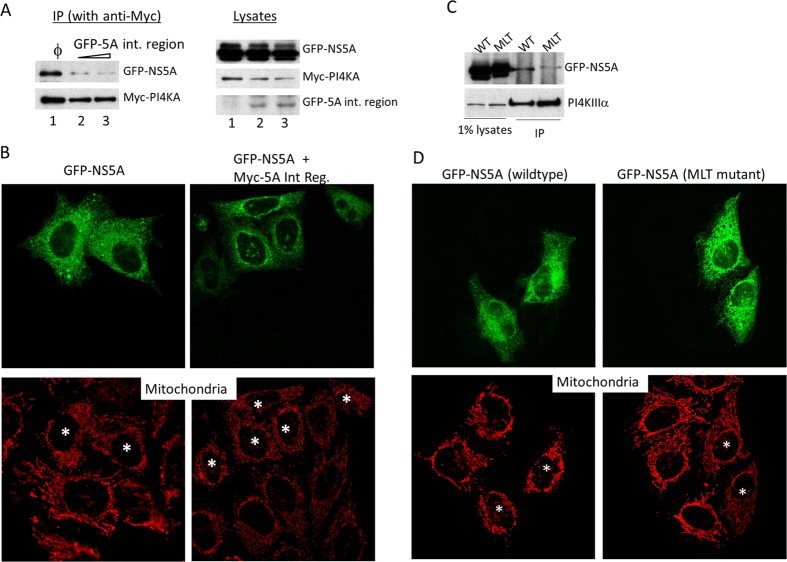
Figure 6. Interaction between NS5A and PI4KA is critical for the NS5A-induced mitochondrial fragmentation.
(A) Disruption of the NS5A-PI4KA interaction by overexpression the NS5A-interacting region of PI4KA in HEK293T cells. The protein interaction between GFP-NS5A and Myc-PI4KA was investigated by immunoblotting after immunoprecipitation using anti-Myc antibody. The interaction between GFP-NS5A and Myc-PI4KA can be disrupted by co-expression of GFP-5A int. region (lanes 2 and 3). (B) Disruption of NS5A-PI4KA interaction can abrogate the mitochondrial fragmentation activity of NS5A. GFP-NS5A alone or in combination with Myc-5A interacting region (Myc-5A Int Reg.) at a ratio of 1:5 was transfected into HEK293 cells which were subsequently stained with mitochondria marker mtHSP70. Transfected cells are indicated with asterisks. (C) NS5A with mutations at the PI4KA binding motif (MLT mutant) shows significantly reduced binding to PI4KA. GFP-NS5A wildtype (WT) or MLT mutant (MLT) were co-expressed with Myc-PI4KA in HEK293T cells and immunoprecipitated with anti-Myc 9E10 antibody (bottom panel). The extent of co-precipitated GFP-NS5A proteins was investigated by immunoblotting (top panel). (D) Overexpression of the NS5A MLT mutant no longer caused mitochondrial fragmentation. GFP-NS5A wildtype or MLT mutant were expressed in HEK293 cells (top panels) and the status of mitochondria was analyzed with immunofluorescence staining of mtHSP70 (bottom panels). Asterisks indicate the corresponding NS5A transfected cells.