Abstract
An Oregon resident returned from a photography trip to Ethiopia with a male Hyalomma truncatum tick attached to the skin on his lower back. The tick was identified morphologically and deposited in the U.S. National Tick Collection housed at Georgia Southern University, Statesboro, Georgia. The public health importance of Hyalomma species of ticks and diagnostic dilemmas with identifying exotic ticks imported into the U.S. are discussed.
CASE REPORT
A 59-year-old previously-healthy male presented to his primary care clinic on December 9, 2013, after discovering a tick on his lower back the preceding day. He had initially mistaken the tick for a skin tag and bluntly removed it. After identifying it as a possible tick, he placed it in a plastic bag with moistened napkin and placed it in the refrigerator. The patient had returned from a photography trip to Ethiopia where he had visited several rural towns and villages approximately five days prior to presentation. After returning to the U.S., he had spent time in California but did not hike nor camp. The patient had reported a self-limiting headache the previous day and chills during the flight home. He was otherwise asymptomatic and had not experienced arthralgias or myalgias. On examination, the patient was afebrile and appeared well. The area surrounding the attachment site was raised, firm, and tender, surrounded by a reddened, concentric rash that was three inches in diameter [Fig. 1]. The case was discussed with the local infectious disease specialist via telephone. Given the patient’s mild symptoms, serologies were not recommended unless he developed worsening symptoms. However, because of the risk of acquiring rickettsial diseases with this species of tick, the patient was started on 100 mg doxycycline twice daily for a total of 21 days.
Fig. 1.
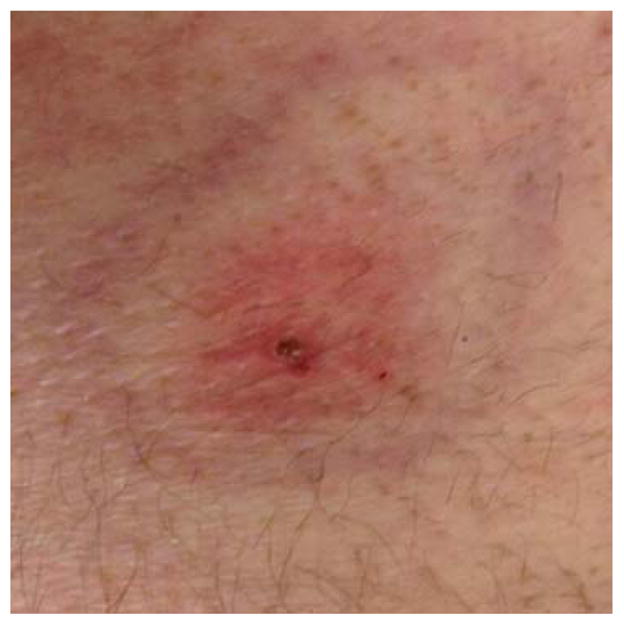
Skin lesion observed at the bite site.
The tick was sent to the Oregon State University Plant Clinic for identification, where it was determined to be a genus or species of hard tick not commonly occurring in the Pacific Northwest. Digital images of the tick were shared with the first author, who made the tentative identification of an adult stage Hyalomma. Diagnostic features supporting the genus-level identification included the presence of eyes, irregular festoons, elongate mouthparts, an inornate dorsal shield, and a characteristic banding pattern seen on the legs of many Hyalomma species [Fig. 2], as well as having the spurs on the forecoxae subequal in length [Fig. 3] (Mathison and Pritt 2014). Digital images were shared with Dr. Dmitry Apanaskevich at the U.S. National Tick Collection (USNTC) at Georgia Southern University who suggested an identification of H. truncatum. The specimen was forwarded to the Centers for Disease Control and Prevention, Division of Parasitic Diseases and Malaria, where the identification of H. truncatum was confirmed based on the following morphologic criteria: a dorsal shield irregularly punctate, denser posteriorly; lateral grooves [Fig. 2] deep and reaching the eyes; subanal plates in-line with the adanal plates [Fig. 3] (Apanaskevich and Horak 2008c). The specimen (a male) was deposited in the USNTC for archiving.
Fig. 2.

Dorsal image of the Hyalomma specimen (male). (EY, eye; LG, lateral groove; FS, festoons).
Fig 3.
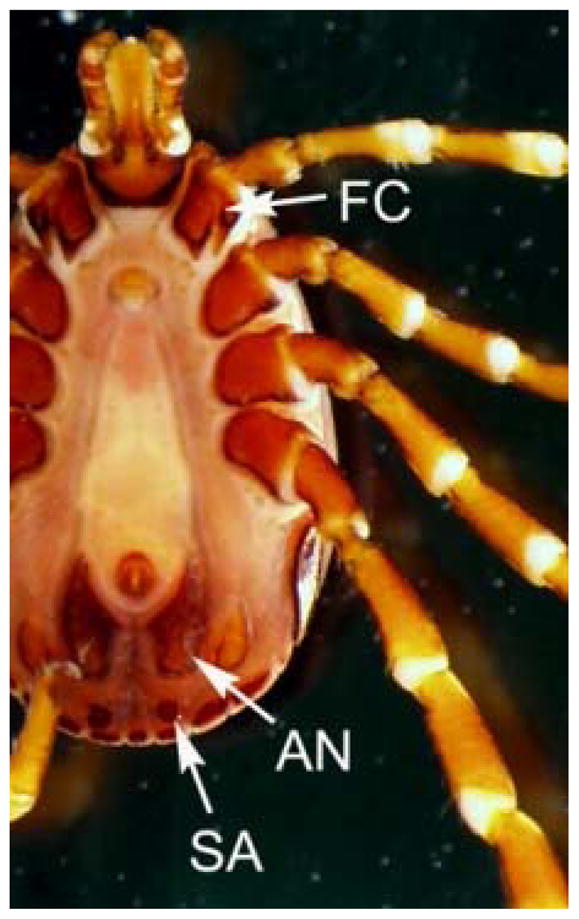
Ventral image of the Hyalomma specimen (male). (FC, spurs on the forecoxa; AN, adanal plate; SA, subanal plate).
DISCUSSION
Hyalomma is one of the most medically-important tick genera in the Old World. Species in this genus have been reported to transmit a variety of viral, bacterial, and parasitic diseases of medical and veterinary importance (Bakheit et al. 2012). One of the most important human viral agents transmitted by Hyalomma spp. is Crimean-Congo hemorrhagic fever (CCHF) virus. Sexually and transovarially transmission of CCHF virus was observed experimentally in Hyalomma truncatum (Gonzalez et al 1992). In addition, extensive documentation exist showing that several tick species transmit this virus in different geographical areas of the world: H. marginatum in southern Russia, Turkey, and the Balkan and Crimean Peninsulas, H. anatolicum in Iran, Pakistan, Turkmenistan, and Tajikistan, H. asiaticum from central Asia to China, and H. rufipes in Africa (Hoogstraal 1979; Bakheit et al. 2012; Goddard 2012). Other important disease agents of humans and their documented vectors include Rickettsia conorii (H. rufipes and H. truncatum), R. aeschlimannii (H. marginatum, H. truncatum, and H. scupense), R. sibirica (H. asiaticum, H. excavatum, and H. truncatum), Anaplasma phagocytophilum (H. lusitanicum), and Coxiella burnetii (H. aegyptium and H. scupense) (Beati et al. 1997; Bakheit et al. 2012; Goddard 2012), and Dugbe virus (Hoogstraal et al. 1981). Kumsa et al. (2014) detected R. aeschlimannii in H. margniatum and H. truncatum in Ethiopia. Paştiu et al. (2012) found H. aegyptium naturally infected with Ehrlichia canis and A. phagocytophilum in Romania. Hyalomma truncatum has been suggested as a possible vector of Rift Valley fever virus (Linthicum et al. 1989; Nchu and Rand 2013). Hyalomma species have also been implicated in tick paralysis in humans (Edussuriya et al. 2003; Gürbüz et al. 2010; Doğan et al. 2012). In general, prophylaxis is not recommended for prevention of rickettsial or viral tickborne infections (Wormser et al. 2006, Bakken et al. 2006). However, a course of ribavirin may be considered for prophylaxis of CCHF in individuals at high risk of severe disease (Appannanavar et al. 2011).
There are scattered reports in the literature of Hyalomma species being imported into the United States, most-commonly on animals and animal products. Mertins and Schlater (1991) documented five species of Hyalomma on ostriches imported from Africa and Europe. Burridge and Simmons (2003) documented H. aegyptium imported on Greek tortoises to New York, Florida, and North Carolina. Becklund (1968) and Keirans and Durden (2001) presented comprehensive overviews of imported ticks into the U.S. Most of the records for Hyalomma spp. are from animals or products made from animals (e.g. trophy hides), but Keirans and Durden (2001) did report one case of H. marginatum being found on a human with travel history to Greece. In the USNTC, there is a specimen of H. truncatum collected from a human in Illinois following travel to Botswana (Dmitry Apanaskevich, pers. comm. 2013). Hyalomma truncatum has a wide host range; Apanaskevich and Horak (2008c) documented seven mammalian hosts for the immature stages and 18 mammalian hosts (including humans) for adults in South Africa. As such, the risk for a person to acquire this tick in an endemic area would be greater than for those species whose normal hosts are tortoises and fossorial or other wild animals that have less human contact.
Because Hyalomma species are not native to North America, medical practitioners and diagnostic microbiologists and parasitologists may not consider them in the differential diagnosis when identifying ticks removed from patients treated in the U.S. Given their morphologic features (elongate mouthparts, eyes present, festoons present), Hyalomma spp. might be misidentified as Amblyomma in most keys to North American ticks. Hyalomma can best be separated from Amblyomma by having festoons of varying size, an inornate dorsal shield (scutum), and spurs on the coxae I roughly equal in length (Mathison and Pritt 2014). Many Hyalomma species also have a characteristic banding pattern on their legs whereby the legs are dark with white maculae at the joints (Fig. 2). Regional keys for Hyalomma are available for the United Kingdom (Arthur 1963), Russia [former U.S.S.R.] (Pomerantzev 1950), Sudan (Hoogstraal 1956), Uganda (Matthysse and Cobo 1987), Saudi Arabia (Hoogstraal et al. 1981), Pakistan and the Indian subcontinent (Kaiser and Hoogstraal 1964), and Iran (Hosseini-Chegeni et al 2013). Recent systematic treatments are available for the subgenera Euhyalomma (Apanaskevich and Horak 2005a, 2005b, 2007, 2008a, 2008b, 2008c, 2008d, 2009, 2010a, 2010b) and Hyalommina (Apanaskevich et al. 2009). As with other ectoparasites, detailed travel history can be important for obtaining a definitive diagnosis of ticks, as well as to help assess the risk of vector-borne diseases.
Acknowledgments
We’d like to thank Dmitry Apanaskevich (USNTC) for assisting with the identification. Rosmarie Kelly (Georgia Department of Health, Atlanta, GA) and William Nicholson (Rickettsial Zoonoses Branch, Division of Vector-Borne Diseases, CDC, Atlanta, GA) kindly reviewed the manuscript.
Contributor Information
Blaine A. Mathison, Email: gqa4@cdc.gov, Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention, Atlanta, GA. 404-718-4103.
William J. Gerth, Oregon State University Plant Clinic, Corvallis, OR.
Bobbi S. Pritt, Mayo Clinic, Rochester, MN.
Stephen Baugh, Legacy Medical Group Northwest, Portland, OR.
References
- Apanaskevich DA, Horak IG. The genus Hyalomma Koch, 1844. I. Reinstatement of Hyalomma (Euhyalomma) glabrum Delpy, 1949 (Acari, Ixodidae) as a valid species with a redescription of the adults, the first description of its immature stages and notes on its biology. Onderstepoort Journal of Veterinary Research. 2005a;73:1–12. doi: 10.4102/ojvr.v73i1.164. [DOI] [PubMed] [Google Scholar]
- Apanaskevich DA, Horak IG. The genus Hyalomma Koch, 1844. II. Taxonomic status if H. (Euhyalomma) anatolicum Koch, 1844 and H. (E.) excavatum Koch, 1844 (Acari, Ixodidae) with redescriptions of all stages. Acarina. 2005b;13:181–197. [Google Scholar]
- Apanaskevich DA, Horak IG. The genus Hyalomma Koch, 1844. III. Redescription of the adults and larvae of H. (Euhyalomma) impressum Koch, 1844 (Acari: Ixodidae) with a first description of its nymph and notes on its biology. Folia Parasitologica. 2007;54:51–58. doi: 10.14411/fp.2007.007. [DOI] [PubMed] [Google Scholar]
- Apanaskevich DA, Horak IG. The genus Hyalomma Koch, 1844. IV. Redescription of all parasitic stages of H. (Euhyalomma) lusitanicum Kock, 1844 and the adults of H. (E.) franchinii Tonelli Rondelli, 1932 (Acari: Ixodidae) with a first description of its immature stages. Folia Parasitologica. 2008a;55:61–74. doi: 10.14411/fp.2008.009. [DOI] [PubMed] [Google Scholar]
- Apanaskevich DA, Horak IG. The genus Hyalomma Koch, 1844: V. Re-evaluation of the taxonomic rank of taxa comprising the H. (Euhyalomma) marginatum complex of species (Acari: Ixodidae) with redescription of parasitic stages and notes on biology. International Journal of Acarology. 2008b;34:13–42. [Google Scholar]
- Apanaskevich DA, Horak IG. The genus Hyalomma Koch, 1844. VI. Systematics of H. (Euhyalomma) truncatum and the closely related species, H. (E.) albiparmatum and H. (E.) nitidum (Acari: Ixodidae) Experimental Applied Acarology. 2008c;44:115–136. doi: 10.1007/s10493-008-9136-z. [DOI] [PubMed] [Google Scholar]
- Apanaskevich DA, Horak IG. The genus Hyalomma Koch, 1844. VII. Redescription of all parasitic stages of H. (E.) dromedarii and H. (E.) schulzei (Acari: Ixodidae) Journal of Medical Entomology. 2008d;45:817–831. doi: 10.1603/0022-2585(2008)45[817:tghvro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Apanaskevich DA, Horak IG. The genus Hyalomma Koch, 1844, IX. Redescription of all parasitic stages of H. (E.) impeltatum Sculze & Schlottke, 1930 and H. (E.) somalicum Tonelli Rondelli, 1935 (Acari: Ixodidae) Systematic Parasitology. 2009;73:199–218. doi: 10.1007/s11230-009-9190-x. [DOI] [PubMed] [Google Scholar]
- Apanaskevich DA, Horak IG. The genus Hyalomma Koch, 1844. X. Redescription of the parasitic stages of H. (Euhyalomma) scupese Schulze, 1919 (=H. detritum Schulze) (Acari: Ixodidae) and notes on its biology. Folia Parasitologica. 2010a;57:69–78. doi: 10.14411/fp.2010.009. [DOI] [PubMed] [Google Scholar]
- Apanaskevich DA, Horak IG. The genus Hyalomma Koch, 1844. XI. Redescription of all stages of H. (Eyhyalomma) asiaticum (Acari: Ixodidae) and notes on its biology. Experimental Applied Acarology. 2010b;52:207–220. doi: 10.1007/s10493-010-9361-0. [DOI] [PubMed] [Google Scholar]
- Apanaskevich DA, Horak IG, Geevarghese G. The genus Hyalomma Koch, 1844. VIII. Redescription of three Hyalommina Schulze, 1919 species (Acari: Ixodidae) from South Asia with notes on their biology. Zootaxa. 2009;2050:31–55. [Google Scholar]
- Appannanavar SB, Mishra B. An update on Crimean Congo Hemorrhagic Fever. Journal of Global Infectious Diseases. 2011;3:285–292. doi: 10.4103/0974-777X.83537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur DR. British ticks. Butterworths; London, United Kingdom: 1963. [Google Scholar]
- Bakheit MA, Latif AA, Vatansever Z, Seitzer U, Ahmed J. Chapter 8. The huge risks due to Hyalomma ticks. In: Mehlhorn H, editor. Arthropods as vectors of emerging diseases. Springer-Verlag; Berlin: 2012. [Google Scholar]
- Bakken JS, Folk SM, Paddock ChD, Bloch KC, Krusell A, Sexton DJ, Buckingham SC, Marshall GS, Storch GA, Dasch GA, McQuiston JH, Swerdlow DL, Dumler JS, Nicholson WL, Walker DH, Eremeeva ME, Ohl CA. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain Spotted Fever, ehrlichiosis, and anaplasmosis --- United States. Morbidity and Mortality Weekly Report. 2006;55:1–27. [PubMed] [Google Scholar]
- Beati L, Meskini M, Thiers B, Raoult D. Rickettsia aeschlimannii sp. nov., a new spotted fever group of rickettsia associated with Hyalomma marginatum ticks. International Journal of Systematic Bacteriology. 1997;47:548–554. doi: 10.1099/00207713-47-2-548. [DOI] [PubMed] [Google Scholar]
- Becklund WW. Ticks of veterinary significance found on imports in the United States. Journal of Parasitology. 1968;54:622–628. [PubMed] [Google Scholar]
- Burridge MJ, Simmons LA. Exotic ticks introduced into the United States on imported reptiles from 1962 to 2001 and their potential roles in the international dissemination of diseases. Veterinary Parasitology. 2003;113:289–320. doi: 10.1016/s0304-4017(03)00060-8. [DOI] [PubMed] [Google Scholar]
- Doğan M, Devge C, Tanriöver Pata YS, Sönmezoğlu M. Case report: facial nerve paralysis due to intra-aural Hyalomma tick infestation. Turiye Parazitologia Derg. 2012;36:254–257. doi: 10.5152/tpd.2012.60. [DOI] [PubMed] [Google Scholar]
- Edussuriya BD, Weilgama DJ. Case reports: intra-aural tick infestations in humans in Sri Lanka. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2003;97:412–413. doi: 10.1016/s0035-9203(03)90072-1. [DOI] [PubMed] [Google Scholar]
- Goddard J. Physician’s guide to arthropods of medical importance. 6. CRC Press; Boca Raton, LA: 2012. [Google Scholar]
- Gonzalez JP, Camicas JL, Cornet JP, Faye O, Wilson ML. Sexual and transovarian transmission of Crimean-Congo haemorrhagic fever virus in Hyalomma truncatum ticks. Research in Virology. 1992;143:23–28. doi: 10.1016/s0923-2516(06)80073-7. [DOI] [PubMed] [Google Scholar]
- Gürbüz MK, Erdoğan M, Doğan N, Birdane L, Cingi C, Cingi E. Case report: isolated facial paralysis with a tick. Turiye Parazitologia Derg. 2010;34:61–64. [PubMed] [Google Scholar]
- Hoogstraal H. African Ixodoidea. I. Ticks of the Sudan (with special reference to Equatoria province and with preliminary reviews of the genera Boophilus, Magaropus, and Hyalomma) U.S. Naval Medical Research Report NM 005 050.29.07 1956 [Google Scholar]
- Hoogstraal H. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. Journal of Medical Entomology. 1979;15:307–417. doi: 10.1093/jmedent/15.4.307. [DOI] [PubMed] [Google Scholar]
- Hoogstraal H, Wassef HY, Büttiker W. Ticks (Acarina) of Saudi Arabia. Fam. Argasidae, Ixodidae. Fauna of Saudi Arabia. 1981;3:25–110. [Google Scholar]
- Hosseini-Chegeni A, Hosseini R, Tavakoli M, Telmadarraiy Z, Abdigoudarzi M. The Iranian Hyalomma (Acari: Ixodidae) with a key to the identification of male species. Persian Journal of Acarology. 2013;2:503–529. [Google Scholar]
- Kaiser MN, Hoogstraal H. The Hyalomma ticks (Ixodoidea, Ixodidae) of Pakistan, India, and Ceylon, with keys to subgenera and species. Acarologia. 6:257–286. [Google Scholar]
- Keirans JE, Durden LA. Invasion: exotic ticks (Acari: Argasidae, Ixodidae) imported into the United States. A review and new records. Journal of Medical Entomology. 2001;38:850–861. doi: 10.1603/0022-2585-38.6.850. [DOI] [PubMed] [Google Scholar]
- Kumsa B, Socolovschi C, Raoult D, Parola P. Spotted fever group rickettsiae in ixodid ticks in Oromia, Ethiopia. Ticks and Tick-borne Diseases. 2014 doi: 10.1016/j.ttbdis.2014.08.001. ePub 2014 Sep 25. pii: S1877-959X(14)00170-8. [DOI] [PubMed] [Google Scholar]
- Linthicum KJ, Logan TM, Bailey CL, Dohm DJ, Moulton JR. Transstadial and horizontal transmission of Rift Valley fever virus in Hyalomma truncatum. American Journal of Tropical Medicine and Hygiene. 1989;41:491–496. doi: 10.4269/ajtmh.1989.41.491. [DOI] [PubMed] [Google Scholar]
- Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clinical Microbiology Reviews. 2014;27:48–67. doi: 10.1128/CMR.00008-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysee JG, Colbo MH. Ixodid Ticks of Uganda. Entomological Society of America; Lanham, MD: 1987. [Google Scholar]
- Mertins JW, Schlater JL. Exotic ectoparasites of ostriches recently imported into the United States. Journal of Wildlife Diseases. 1991;27:180–182. doi: 10.7589/0090-3558-27.1.180. [DOI] [PubMed] [Google Scholar]
- Nchu F, Rand A. Rift Valley fever outbreaks: possible implication of Hyalomma truncatum (Acari: Ixodidae) African Journal of Microbiology Research. 2013;7:3891–3894. [Google Scholar]
- Paştiu AI, Matei IA, Mihalca AD, D’Amico G, Dumitrache MO, Kalmár Z, Sándor A, Lefkaditis M, Gherman CM, Cozma V. Zoonotic pathogens associated with Hyalomma aegyptium in endangered tortoise: evidence for host-switching behavior in ticks? Parasites and Vectors. 2012;5:301–306. doi: 10.1186/1756-3305-5-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantzev BI. Arachnida. 2. IV. Zoological Institute of the Academy of Sciences; USSR, Moscow, Russia: 1950. Ixodid ticks (Ixodidae). Fauna of the USSR. [Google Scholar]
- Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. The Clinical Assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clinical Infectious Diseases. 2006;43:1089–134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]


