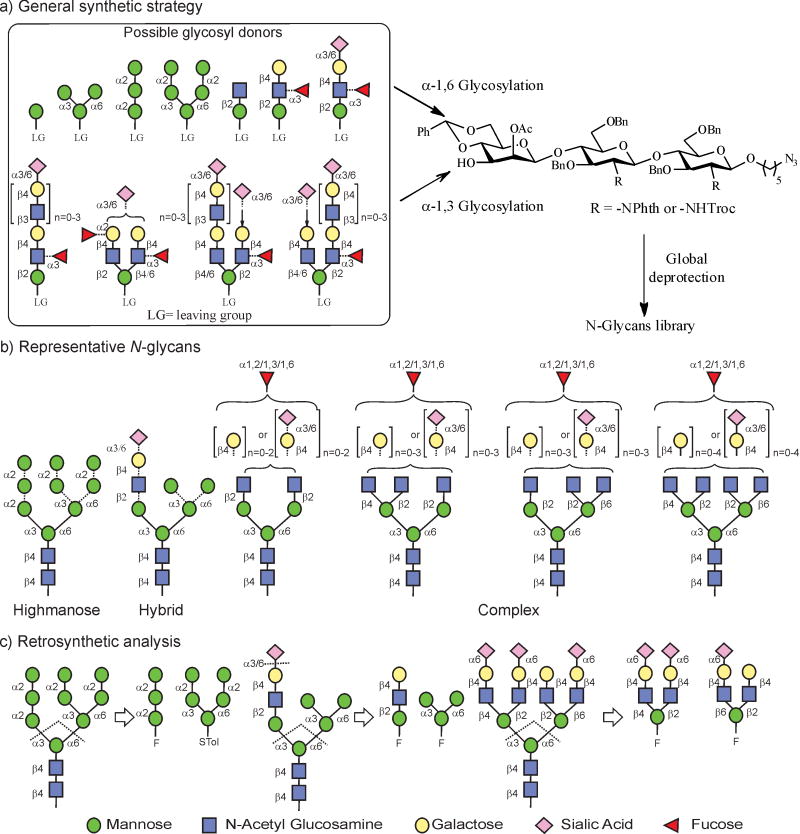
Figure 1. A general strategy for the modular synthesis of gp120-related N-glycans.
Due to the large number of possibilities in glycosidic linkages generating a huge diversity of structures (around 20,000), especially from the GlcNAc residues to the non-reducing end, a modular approach is necessary to minimize the reaction steps and create enough diversity to reflect the nature of N-glycosylation. a, Synthesis of high-mannose-, hybrid- and complex-type N-glycans through regio- and stereoselective glycosidation of orthogonally protected core trisaccharide at the O3 and O6 positions with a modular set of diverse glycosyl donors. b, Representative N-glycans that can be generated by this strategy. c, Retrosynthetic disconnections of high-mannose-, hybrid- and complex-type glycans, showing the building blocks required for assembly.