Abstract
Yersinia pestis, the agent of plague, requires the Ail (attachment invasion locus) outer membrane protein to survive in the blood and tissues of its mammalian hosts. Ail is important for both attachment to host cells and for resistance to complement-dependent bacteriolysis. Previous studies have shown that Ail interacts with components of the extracellular matrix, including fibronectin, laminin and heparan sulfate proteoglycans, and with the complement inhibitor C4b-binding protein. Here, we demonstrate that Ail-expressing Y. pestis strains bind vitronectin – a host protein with functions in cell attachment, fibrinolysis and inhibition of the complement system. The Ail-dependent recruitment of vitronectin resulted in efficient cleavage of vitronectin by the outer membrane Pla (plasminogen activator protease). Escherichia coli DH5α expressing Y. pestis Ail bound vitronectin, but not heat-treated vitronectin. The ability of Ail to directly bind vitronectin was demonstrated by ELISA using purified refolded Ail in nanodiscs.
Introduction
Yersiniae are Gram-negative bacteria of the family Enterobacteriaceae (Putzker et al., 2001). The genus Yersinia includes three species associated with human disease. The enteropathogenic yersiniae (Yersinia enterocolitica and Yersinia pseudotuberculosis) are associated with gastrointestinal disease and are spread via the faecal–oral route. In contrast, Yersinia pestis is the agent of plague and is transmitted via the bite of infected fleas (bubonic plague or septicaemic plague) or via aerosols (pneumonic plague) (Perry & Fetherston, 1997). The three human pathogenic yersiniae share a number of critical virulence attributes including a virulence plasmid pCD1-encoded type III secretion system (T3SS) (Forsberg et al., 1994) and an Ail (attachment invasion locus) outer membrane protein (Miller et al., 1990). The T3SS mediates the cell-contact-dependent injection of Yop (Yersinia outer protein) effectors into targeted host cells. The injected Yop effectors function to block bacterial phagocytosis and to suppress the production of pro-inflammatory cytokines (Viboud & Bliska, 2004). Importantly, the specificity of the Yersinia–host cell interaction is largely determined by bacterial adhesins that recognize defined host cell receptors (Simonet et al., 1996). The enteropathogenic yersiniae express two dominant adhesins/invasins (YadA and Invasin) that are required for efficient cell attachment and subsequent Yop injection (Grosdent et al., 2002). Y. pestis does not express YadA or Invasin (Rosqvist et al., 1988; Simonet et al., 1996; Skurnik & Wolf-Watz, 1989), but expresses other adhesins, including Ail, Pla (plasminogen activator protease) and pH6 antigen (Kolodziejek et al., 2007; Lindler & Tall, 1993; Sodeinde et al., 1992). Ail is expressed by all three human pathogenic yersiniae, but appears to play a more significant role in the virulence of Y. pestis, in part due to the lack of functional YadA and Invasin (Felek et al., 2010; Hinnebusch et al., 2011; Wachtel & Miller, 1995). Importantly, Y. pestis Ail has established roles in cell invasion, cell attachment, Yop injection, serum (complement) resistance and virulence (Bartra et al., 2008; Felek et al., 2010; Hinnebusch et al., 2011; Kolodziejek et al., 2010).
Structural studies have demonstrated that Y. pestis Ail (Marassi et al., 2015; Yamashita et al., 2011) forms an eight-stranded antiparallel β-barrel with four extracellular loops that closely resembles the structure of Escherichia coli OmpX (Vogt & Schulz, 1999). The extracellular loops mediate interactions with components of the extracellular matrix (ECM) (fibronectin, laminin and heparan sulfate proteoglycans) and complement system [C4b-binding protein (C4BP)] that contribute to bacterial attachment/invasion and serum resistance, respectively (Ding et al., 2015; Ho et al., 2014; Tsang et al., 2010; Yamashita et al., 2011). Co-crystallization studies with a heparin analogue in conjunction with site-directed mutagenesis identified two heparin-binding sites (HBS) on Ail formed by residues from extracellular loops 2 and 3 (HBS-1) and extracellular loop 1 (HBS-2) (Yamashita et al., 2011). Mutagenesis studies performed on Y. enterocolitica Ail indicate that residues critical for both cell attachment and serum resistance map to extracellular loops 2 and 3, and appear to cluster around a hydrophobic cleft on the extracellular surface of Ail – a potential site for binding of ECM proteins and/or C4BP (Miller et al., 2001; Yamashita et al., 2011).
Previous studies have demonstrated that Y. pestis Ail directly binds the ECM proteins fibronectin and laminin, as well as the complement regulatory component C4BP. Another study suggested that Y. pestis can recruit vitronectin to its cell surface (Duensing et al., 1999). Bacterial pathogens that recruit ECM proteins as well as C4BP and/or Factor H to their cell surface, such as Haemophilus influenzae (Hallström et al., 2007), Neisseria gonorrhoeae (Ram et al., 1999), Neisseria meningitidis (Lewis & Ram, 2014) and Moraxella catarrhalis (Bernhard et al., 2014; Nordström et al., 2004), have also been shown to recruit vitronectin (Attia et al., 2006; Duensing & van Putten, 1997; Hallström et al., 2006, 2011; Sa E Cunha et al., 2010), indicating that acquisition of vitronectin can be advantageous to pathogens even in the presence of other ECM proteins and complement regulatory factors. Vitronectin is a multifunctional glycoprotein found abundantly in serum and the ECM (Singh et al., 2010). Circulating vitronectin is normally a monomer, whereas cell-bound vitronectin is typically multimeric. Vitronectin plays a critical role in many biological processes, including cell adhesion, fibrinolysis, cell migration and regulation of membrane attack complex (MAC) formation. Bacterial pathogens bind vitronectin to their surface in order to enhance adhesion to host cells and tissues as well as to protect them from MAC-mediated lysis (Singh et al., 2010). In this study, we demonstrate that Y. pestis actively recruits vitronectin to its surface. Furthermore, we demonstrate that the acquisition of vitronectin is dependent upon the Ail outer membrane protein.
Methods
Bacterial strains and growth conditions
All Y. pestis strains used in this study, including KIM5 (pPCP1+; pCD1+), KIM5 Δail (Bartra et al., 2008), KIM8-E (pPCP1− ; pCD1+ ΔyopE–sycE : : dhfr) (Bartra et al., 2006) and KIM8-E Δail (Bartra et al., 2008) are Pgm− and avirulent from peripheral routes of infection (Une & Brubaker, 1984). These strains and their derivatives were routinely grown in heart infusion broth (HIB) supplemented with 2.5 mM CaCl2 or on Tryptose Blood Agar Base plates (Difco) at 27 or 37 °C. In Yop secretion assays, Y. pestis strains were grown in TMH media as described previously (Bartra et al., 2006). E. coli DH5α (Cambau et al., 1993) and derivatives were grown at 37 °C in LB or HIB. When appropriate, media was supplemented with kanamycin (25 μg ml–1) or ampicillin (50 μg ml–1).
Construction of plasmids pFLAG-Ail and pFLAG-OmpX
DNA fragments encoding the mature portion of Y. pestis Ail (y1324; residues 24–194) and OmpX (y1682; residues 25–176) were amplified with oligonucleotides Ail-HindIII-F, Ail-BglII-R, OmpX-HindIII-F and OmpX-BglII-R. The resulting DNA fragments were digested with HindIII and BglII, and inserted into plasmid pFLAG-ATS (Sigma), generating plasmids pFLAG-Ail and pFLAG-OmpX, which direct the expression of N-terminal FLAG-tagged Ail and OmpX.
Bacterial binding of vitronectin
Bacteria were grown in HIB containing 2.5 mM CaCl2 for 5 h at 37 °C. HIB-grown bacteria were washed in ice-cold PBS and suspended in PBS to OD620 5.0. Washed bacteria (250 μl) were added to an equal volume of normal human sera (NHS), heat-inactivated sera (HIS) or purified vitronectin (50 μg ml–1) (Sigma). Binding reactions were incubated for 2 h at 4 °C or 1 h at 37 °C. Bacteria and co-sedimenting proteins were pelleted by centrifugation at 10 000 g for 5 min at 4 °C, washed three times with 1 ml ice-cold PBS containing 0.05 % Tween-20 and lysed by boiling in 100 μl SDS-PAGE sample buffer (50 mM Tris/HCl, 2 % SDS, 5 % glycerol, 1 % β-mercaptoethanol, pH 6.8). Bacterial lysates and co-sedimenting proteins were subjected to SDS-PAGE and immunoblot analysis with mouse monoclonal anti-vitronectin (VIT-2; Sigma), rabbit polyclonal anti-vitronectin (Sigma), anti-Ail (Ding et al., 2015) or anti-FLAG M2 (Sigma) antibodies.
Proteolysis of vitronectin by Pla-expressing Y. pestis
Bacteria were grown in HIB containing 2.5 mM CaCl2 for 5 h at 37 °C. HIB-grown bacteria were washed in ice-cold PBS and suspended in PBS to OD620 10.0. Washed bacteria (300 μl) were added to an equal volume of purified vitronectin (50 μg ml–1). Reactions were incubated for 4 h at 37 °C. Samples (50 μl) were removed at 0, 0.5, 1, 2, 3 and 4 h and placed on ice. Samples were subjected to SDS-PAGE and immunoblot analysis with a mouse monoclonal anti-vitronectin antibody.
Flow cytometric analysis of vitronectin binding
Flow cytometry to detect complement component binding to bacteria was performed as described previously (Ram et al., 2001). Briefly, 3 × 107 bacteria (Y. pestis KIM5 or KIM5 Δail) were incubated with purified human monomeric vitronectin (Innovative Research) or C4BP (both from Complement Technologies) at a final concentration of 5 μg ml–1 for 1 h at 37 °C. After three washes, antibodies against each of the proteins (anti-vitronectin mAb) (Quidel) and anti-C4BP mAb 104 (Härdig et al., 1997) were added (1 : 100 dilution in Hank's balanced salt solution) followed by FITC-conjugated anti-mouse IgG. Data were analysed using FlowJo 7.2.5 data analysis software (TreeStar).
Binding of purified Ail-containing nanodiscs to vitronectin
The preparation of recombinant C-terminal His-tagged Ail (Ail-His) and its incorporation in lipid bilayer nanodiscs was as described previously (Ding et al., 2015). ELISAs were performed with antibody-conjugated horseradish peroxidase and its substrate o-phenylenediamine (Pierce) added at a concentration of 0.5 mg ml–1 in stable peroxide substrate buffer (Thermo Scientific). Human plasma fibronectin (Sigma; F2006) or vitronectin (Sigma) was coated on 96-well plates (Nunc) at a concentration of 20 μM in PBS, which corresponds to 5 μg fibronectin ml–1 and 0.6 μg vitronectin ml–1. After coating overnight at 4 °C, the wells were washed three times with PBS, then blocked with Tris-buffered saline (TBS) containing 3 % milk for 2 h at room temperature, and finally washed with TBST (TBS with 0.05 % Tween-20 included to prevent non-specific binding). Incremental concentrations of nanodisc-incorporated Ail-His in TBST were added to the coated wells and the plates were incubated at 37 °C for 3 h and then at 4 °C overnight. Bound Ail-His was probed by adding mouse anti-His mAb (Qiagen; 1 : 100 dilution in TBST-milk) to the wells and incubating for 2 h at room temperature. Unbound primary antibody and Ail were removed by washing three times with TBST-milk. Then, secondary goat anti-mouse antibody conjugated to horseradish peroxidase (Sigma; 1 : 10 000 dilution) was added, the plates were then incubated for 1 h at room temperature, and finally washed three times with PBS and once with TBS, before adding fresh o-phenylenediamine to develop A490.
Results
Binding of vitronectin to Ail-expressing Y. pestis
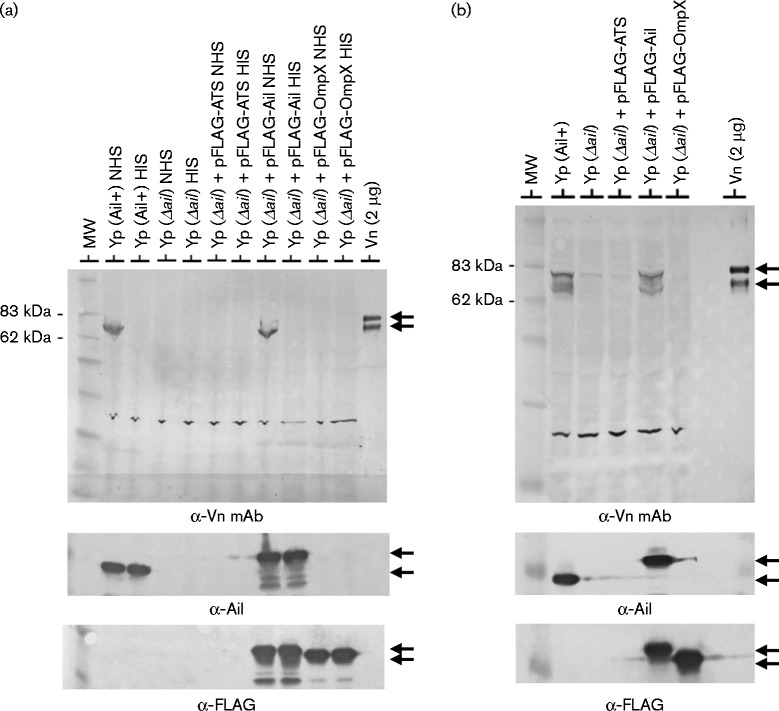
Y. pestis Ail has been shown to interact with fibronectin, laminin and C4BP; however, the interaction of Ail with vitronectin has not been examined. To determine whether Y. pestis interacts with serum vitronectin, Y. pestis KIM8-E (Pla– Ail+) and Y. pestis KIM8-E Δail (Pla– Ail–) were incubated with NHS or HIS at 4 °C and evaluated for co-sedimentation of vitronectin with the bacteria by immunoblotting with a monoclonal anti-vitronectin antibody (Fig. 1a). Immunoreactive bands corresponding in size to the characteristic 75 and 65 kDa bands of vitronectin were observed in samples containing the Ail-expressing bacteria (KIM8-E) incubated with NHS; however, no vitronectin bands co-sedimented with the ail deletion mutant (KIM8-E Δail) or with bacteria incubated with HIS. Complementation of the ail deletion mutant with plasmid pFLAG-Ail (Bartra et al., 2008), which expresses functional N-terminal FLAG-tagged Ail, restored binding of vitronectin (Fig. 1a). Providing the pFLAG-ATS vector or pFLAG-OmpX, which expresses FLAG-tagged Y. pestis OmpX (y1682), did not re-establish vitronectin binding. These results suggested that Y. pestis recruits vitronectin to its surface in an Ail-dependent manner and that heat inactivation of NHS denatures or alters the structure of vitronectin in a manner that interferes with its binding to Ail.
Fig. 1.
Y. pestis expressing Ail binds vitronectin. (a) Co-sedimentation of vitronectin from NHS or HIS with Y. pestis KIM8-E (Yp Ail+) and Y. pestis KIM8-E Δail (Yp Δail). Bacteria were incubated with 50 % NHS or HIS at 4 °C for 2 h. Washed cells and co-sedimenting proteins were subjected to SDS-PAGE and immunoblotting with VIT-2 anti-vitronectin mAb (α-Vn mAb), anti-FLAG M2 mAb (α-FLAG) or anti-Ail (α-Ail) antisera. The 65 and 75 kDa vitronectin bands are marked by arrows. Complementation of Y. pestis KIM8-E Δail with plasmid pFLAG-Ail restores expression of Ail and binding of vitronectin. In contrast, expression of the pFLAG-ATS vector or pFLAG-OmpX does not restore vitronectin binding. (b) Co-sedimentation of purified monomeric vitronectin with Y. pestis KIM8-E (Yp Ail+) and Y. pestis KIM8-E Δail (Yp Δail). Bacteria were incubated with pure vitronectin (50 μg ml–1) at 37 °C for 1 h and washed cells subjected to immunoblot analysis with anti-vitronectin mAb (α-Vn mAb), FLAG M2 mAb (α-FLAG) or anti-Ail (α-Ail) antisera. MW, molecular mass standard.
Binding of purified vitronectin to Ail-expressing Y. pestis
Vitronectin is known to interact with other serum components such as plasminogen activator inhibitor (PAI)-1 (Salonen et al., 1989) as well as complement components C5b–7 and C9 (Milis et al., 1993). To verify that the interaction of vitronectin with Ail occurs independent of these and other serum components, we examined the binding of purified vitronectin to Y. pestis. Purified vitronectin co-sedimented with Y. pestis expressing native Ail (KIM8-E) or FLAG-tagged Ail (Fig. 1b), but did not interact with KIM8-E Δail or KIM8-E Δail carrying pFLAG-ATS or pFLAG-OmpX, indicating that Ail-expressing Y. pestis recognize and directly bind vitronectin.
Binding of vitronectin to Ail-expressing E. coli
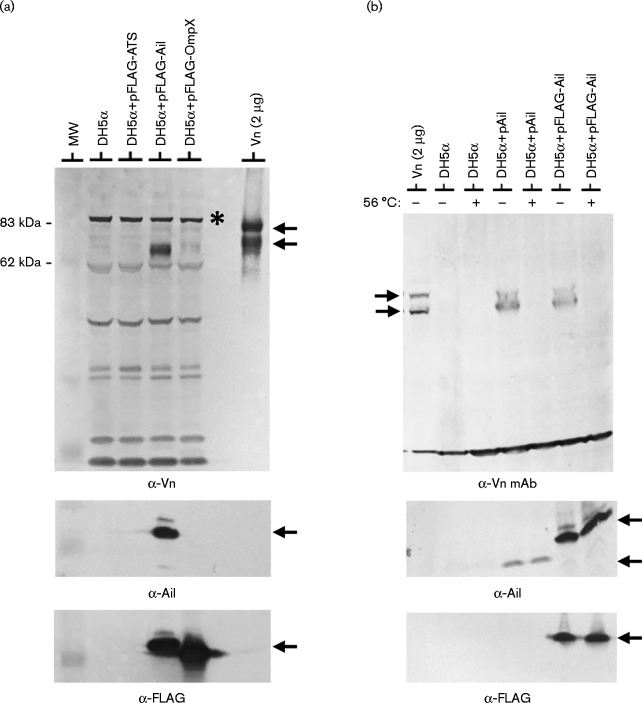
To confirm that the binding of vitronectin to Y. pestis Ail is direct and is not dependent upon other Y. pestis surface components, we examined the binding of purified vitronectin to E. coli DH5α as well as to E. coli DH5α expressing FLAG-tagged Ail (Fig. 2a). Vitronectin did not bind to E. coli DH5α or DH5α carrying pFLAG-ATS or pFLAG-OmpX, but did co-sediment with E. coli DH5α expressing FLAG-tagged Ail, confirming that Ail binds vitronectin independent of other Y. pestis-specific surface components or structures. Heat-treated (56 °C for 30 min) purified vitronectin did not interact with E. coli DH5α or DH5α expressing native Ail or FLAG-tagged Ail (Fig. 2b), confirming that heat treatment alters the conformation of vitronectin in a manner that prevents recognition by Ail.
Fig. 2.
E. coli expressing Y. pestis Ail binds vitronectin. (a) Co-sedimentation of purified monomeric vitronectin with E. coli DH5α expressing FLAG-tagged Ail. Bacteria were incubated with pure vitronectin (50 μg ml–1) at 37 °C for 1 h and washed cells subjected to immunoblot analysis with anti-vitronectin antisera (α-Vn), FLAG M2 mAb (α-FLAG) or anti-Ail (α-Ail) antisera. The 65 and 75 kDa vitronectin bands are marked by arrows. Y. pestis KIM8-E Δail carrying plasmid pFLAG-Ail expressed FLAG-tagged Ail and bound vitronectin. In contrast, providing the pFLAG-ATS vector or pFLAG-OmpX did not result in vitronectin binding. A cross-reactive protein that is not vitronectin is indicated with an asterisk. MW, molecular mass standard. (b) Co-sedimentation of purified monomeric vitronectin with E. coli DH5α expressing Y. pestis Ail or FLAG-tagged Ail. Bacteria were incubated with purified vitronectin (50 μg ml–1) or heat-treated vitronectin (56 °C 30 min; 50 μg ml–1) at 37 °C for 1 h and washed cells subjected to immunoblot analysis with anti-vitronectin mAb (α-Vn mAb), FLAG M2 mAb (α-FLAG) or anti-Ail (α-Ail) antisera.
Binding of vitronectin to Pla-expressing Y. pestis
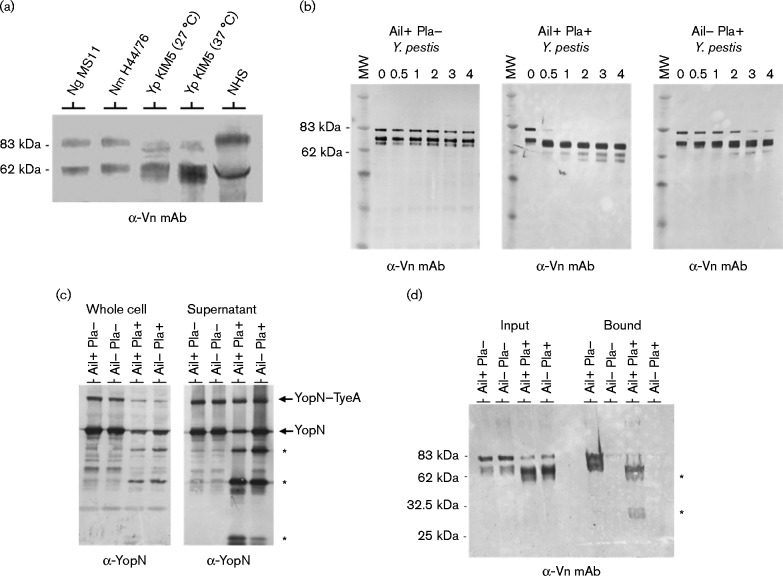
Previous studies by Haiko et al. (2010) demonstrated that Y. pestis Pla degrades the serum PAI-1/vitronectin complex. To investigate the role of Pla in the interaction of Ail with vitronectin, the NHS vitronectin-binding profile of (Pla+ Ail+) Y. pestis KIM5 was compared with those of N. gonorrhoeae and N. meningitidis – pathogens that also recruit vitronectin, either directly, through the protein Opc in the case of N. meningitidis (Sa E Cunha et al., 2010), or indirectly, through bridging by select glycosaminoglycans in the case of N. gonorrhoeae (Duensing et al., 1999) (Fig. 3a). Y. pestis appeared to preferentially recruit the 65 kDa form of serum vitronectin (found in serum as part of a disulfide-linked dimer of the 65 kDa chain and 10 kDa chain that form after endogenous cleavage of 75 kDa vitronectin) rather than the 75 kDa monomeric protein, whereas N. gonorrhoeae and N. meningitidis appeared to recognize both forms of vitronectin approximately equally. The 75 kDa form of vitronectin also appeared to be degraded in the presence of Pla, resulting in several new anti-vitronectin antibody-reactive bands, a process that likely contributes to the apparent preferential binding of the 65 kDa form of serum vitronectin by Ail+ Pla+Y. pestis KIM5.
Fig. 3.
Binding and degradation of vitronectin by Y. pestis. (a) Co-sedimentation of vitronectin from NHS with N. gonorrhoeae (Ng), N. meningitidis (Nm) and Y. pestis (Yp) KIM5 (strain JG150; grown at 27 or 37 °C). Bacteria were incubated with 50 % NHS at 37 °C for 1 h and washed cells were subjected to immunoblot analysis with anti-vitronectin mAb (α-Vn mAb). (b) Degradation of vitronectin by Ail+ Pla+ (Y. pestis KIM5), Ail– Pla+ (KIM5 Δail) and Ail+ Pla– (KIM8-E) was determined by SDS-PAGE and immunoblot analysis with anti-vitronectin mAb (α-Vn mAb) over a 4 h time course. MW, molecular mass standard. (c) Degradation of secreted YopN by Pla-expressing Y. pestis. Ail+ Pla– (KIM8-E), Ail– Pla– (KIM8-E Δail), Ail+ Pla+ (KIM5) and Ail– Pla+ (KIM5 Δail) Y. pestis were grown in TMH media for 5 h at 37 °C. Whole bacterial cells (Whole cell) and culture supernatants (Supernatant) were separated by centrifugation and subjected to immunoblot analysis with antisera specific for YopN (α-YopN). Pla-generated YopN degradation products are indicated with an asterisk. YopN–TyeA is a functional secreted YopN–TyeA hybrid protein that is the result of a+1 translational frameshift event (Ferracci et al., 2004). (d) Binding of intact and degraded vitronectin to Ail+ Pla– (KIM8-E), Ail– Pla– (KIM8-E Δail), Ail+ Pla+ (KIM5) and Ail– Pla+ (KIM5 Δail) Y. pestis. Bacteria were incubated with vitronectin (25 μg ml–1) for 1 h at 37 °C and an aliquot of each sample removed (Input). The input material as well as washed cells with co-sedimenting proteins (Bound) were subjected to immunoblot analysis with anti-vitronectin mAb (α-Vn mAb). Pla-generated vitronectin degradation products are indicated with an asterisk.
Proteolytic degradation of vitronectin by Pla is facilitated by Ail
The co-sedimentation experiments with Pla-expressing Y. pestis KIM5 revealed that several new anti-vitronectin antibody-reactive bands appeared following incubation of Y. pestis with NHS at 37 °C, suggesting that the outer membrane Pla protease may cleave serum vitronectin. To determine whether Pla degrades isolated vitronectin and whether Ail contributes to this process, Ail+ Pla+ (KIM5), Ail– Pla+ (KIM5 Δail) and Ail+ Pla– (KIM8-E) Y. pestis were incubated with purified vitronectin at 37 °C and degradation of vitronectin measured by SDS-PAGE and immunoblot analysis over a 4 h time course (Fig. 3b). Y. pestis KIM5 (Ail+ Pla+) rapidly degraded the purified vitronectin; in contrast, Y. pestis KIM8-E (Ail+ Pla–) did not degrade vitronectin, confirming that vitronectin is a Pla substrate. Y. pestis KIM5 Δail (Ail– Pla+) also degraded vitronectin, but at a much slower rate than the Ail+ Pla+ strain. These results suggested that Ail plays a critical role in facilitating the degradation of vitronectin, possibly by recruiting vitronectin to the bacterial surface. Alternatively, the decreased degradation of vitronectin observed in the Ail– Pla+ strain could be due a decrease in Pla activity associated with the absence of Ail. To examine this possibility, the degradation of the secreted Pla substrate YopN was measured as an independent means of assessing Pla activity (Fig. 3c). The secreted YopN protein was stable in both Pla– strains. In contrast, typical Pla-dependent YopN degradation products were observed in both Pla+ strains; however, the degradation of YopN was reduced in the Ail– Pla+ strain, resulting in 1.8-fold more full-length YopN in the Ail– Pla+ strain than in the Ail+ Pla+ strain. These results suggest that Pla activity is increased in the presence of Ail or that Ail recruits both YopN and vitronectin to the bacterial surface, thus facilitating their degradation.
Y. pestis Ail binds the Pla-cleaved fragments of vitronectin
To determine whether Ail binds the vitronectin peptide fragments generated by Pla, Y. pestis KIM8-E (Pla– Ail+), KIM8-E Δail (Pla– Ail–), KIM5 (Ail+ Pla+) and KIM5 Δail (Pla+ Ail–) were incubated with purified vitronectin at 37 °C for 1 h to allow Pla-dependent degradation of vitronectin, and evaluated for co-sedimentation of vitronectin and/or vitronectin peptides with the bacteria by immunoblotting with a monoclonal anti-vitronectin antibody (Fig. 3d). As expected, no vitronectin peptides co-sedimented with the Ail– bacteria. In contrast, immunoreactive bands corresponding in size to the characteristic 75 and 65 kDa forms of vitronectin co-sedimented with Ail+ Pla– bacteria. Surprisingly, essentially all of the vitronectin peptides generated by Pla appeared to co-sediment with the Ail+ Pla+ bacteria, indicating that the degradation of vitronectin by Pla does not disrupt the Ail–vitronectin interaction.
Binding of vitronectin to Ail-expressing Y. pestis measured by flow cytometry
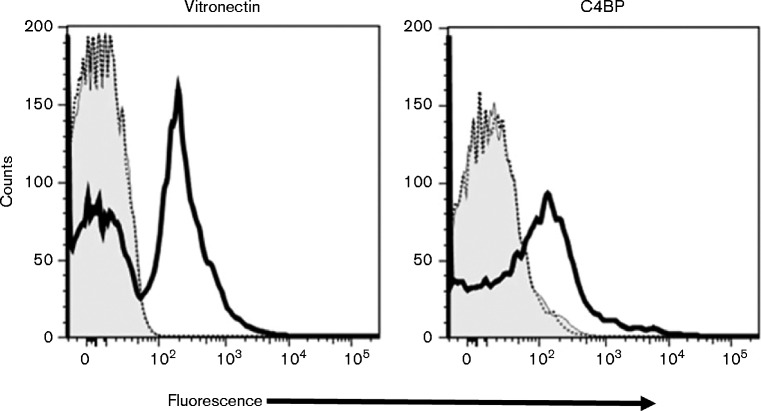
To independently confirm a role for Ail in recruiting vitronectin to the Y. pestis bacterial surface, the capacity of a Pla+Y. pestis ail deletion mutant (KIM5 Δail) and its Pla+ parent strain (KIM5) to bind vitronectin was analysed by flow cytometry. Bacteria were incubated with purified human C4BP or vitronectin and processed for flow cytometry (Fig. 4). Both C4BP and vitronectin bound to the parent strain, but not to the ail deletion mutant. A portion of the KIM5 bacteria did not bind vitronectin in this assay, which may reflect cleavage of vitronectin by Pla and subsequent loss of antibody binding. Overall, these studies confirmed that Y. pestis recruits the multifunctional vitronectin glycoprotein to its surface via an Ail-dependent mechanism.
Fig. 4.
Binding of vitronectin and C4BP to Y. pestis KIM5 and KIM5 Δail. Binding of purified human C4BP and vitronectin to Y. pestis KIM5 (solid black line) and KIM5 Δail (dashed line) was evaluated by flow cytometry. The concentration of each of the proteins in the reaction mixture was 5 μg ml–1. Binding of primary and secondary antibodies (no added complement component) to the bacteria represents the background (grey histogram). The x-axis represents fluorescence on a five-decade log10 scale and the y-axis represents the number of events. One representative experiment of two reproducibly repeated experiments is shown.
Binding of vitronectin by purified recombinant Ail
To further confirm the presence of a direct interaction between Ail and vitronectin, we examined the binding of purified recombinant Ail and vitronectin by ELISA. Previously, we showed that the structure and fibronectin binding activity of Y. pestis Ail can be reconstituted in phospholipid bilayer nanodiscs enabling structural and activity studies to be performed in identical samples (Ding et al., 2015). Nanodiscs are detergent-free, and provide a phospholipid bilayer environment that recapitulates the key anisotropic physical and chemical properties of biological membranes (Nath et al., 2007; Ritchie et al., 2009). Consistent with the pull-down assays performed with whole bacterial cells, Ail exhibits concentration-dependent binding to vitronectin-coated plates, with an ELISA profile similar to that observed for binding to fibronectin-coated plates (Ding et al., 2015) (Fig. 5). In contrast, assays performed with empty nanodiscs yielded no ELISA signal, confirming that the binding signals are due to Ail in these experiments.
Fig. 5.
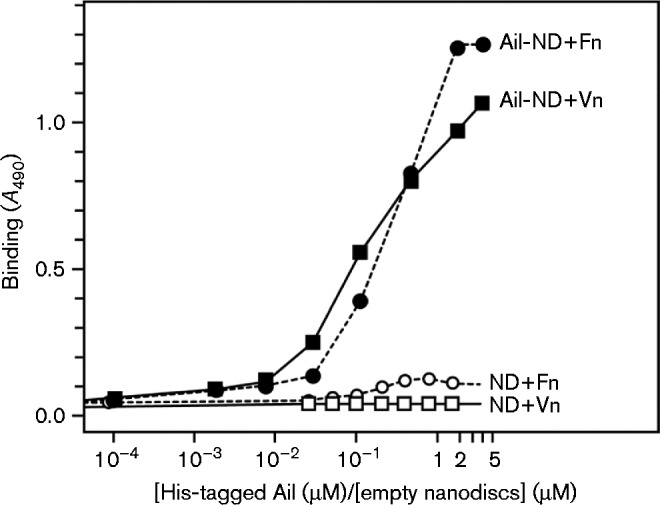
Vitronectin-binding activity of purified Ail detected by ELISA. Purified refolded Ail-His in nanodiscs) (at 0.06 μM Ail concentration) binds fibronectin (Ail-ND+Fn) and vitronectin (Ail-ND+Vn). In contrast, empty nanodiscs bind neither fibronectin (ND-Fn) nor vitronectin (ND-Vn). Ail-His nanodiscs or empty nanodiscs were added at increasing concentrations to vitronectin- and fibronectin-coated plates and incubated overnight. Binding was detected by ELISA using a mouse anti-His antibody. Each point in each dataset represents the mean of three experiments.
Discussion
Y. pestis is a pathogen whose goal is to produce high-level septicaemia in its host in order to facilitate transmission via its flea vector (Lorange et al., 2005). To survive in the blood and tissues of its host, Y. pestis must avoid engulfment by host phagocytic cells and destruction via the complement system. We have previously shown that the Ail outer membrane protein is essential for Y. pestis to avoid complement-dependent bacteriolysis (Bartra et al., 2008). In addition, Ail has been demonstrated to play a critical role in the cell adhesion-mediated selection of cells for injection by the T3SS (Felek & Krukonis, 2009; Felek et al., 2010). However, the mechanisms by which Ail facilitates adhesion to host cells and mediates complement resistance are not well understood. Ail has been shown to bind the ECM proteins fibronectin and laminin, which contribute to bacterial adhesion, and to the complement inhibitor C4BP, which contributes to complement resistance (Ho et al., 2014; Tsang et al., 2010; Yamashita et al., 2011). Here, we demonstrate that Y. pestis Ail also actively recruits the multifunctional glycoprotein vitronectin to the bacterial surface. Numerous other bacterial pathogens recruit vitronectin to both increase adherence to eukaryotic cells and/or evade complement-dependent killing (Attia et al., 2006; Duensing & van Putten, 1997; Hallström et al., 2009, 2011; Sa E Cunha et al., 2010). Vitronectin contains an Arg–Gly–Asp (RGD) sequence for interaction with host αvβ3 or αvβ5 integrins (Lössner et al., 2009). Thus, bacterial-bound vitronectin can function as a bridge that links bacteria to select integrin-expressing cells. Vitronectin also binds the C5b–7 complex of complement and prevents its membrane insertion as well as complement component C9 to further inhibit MAC assembly (Milis et al., 1993). Serum vitronectin is also a critical component of the coagulation pathway, since its interaction with PAI-1 is essential for stabilizing the enzyme's function as a key regulatory brake in the conversion of plasminogen to plasmin during fibrinolysis. Thus, the recruitment of vitronectin by Y. pestis could play a key role in bacterial attachment to host cells, complement resistance and inhibiting coagulation by enhancing fibrinolysis. Defining the biological role of the Ail–vitronectin interaction in bacterial attachment and/or complement resistance is complicated by the multitude of substrates recognized by Ail and the lack of defined Ail mutants defective in binding to individual substrates. Determination of the precise fragments or domains of its host ligands involved in binding to Ail could provide important information in this regard.
In several experiments (Figs 1a, 2a, b and 3a), Ail-expressing bacteria appeared to preferentially bind the 65 kDa form of serum vitronectin, whereas other pathogens such as N. gonorrhoeae and N. meningitidis bound both forms of vitronectin approximately equally (Fig. 3a). The 65 kDa form of vitronectin is normally generated by proteolytic processing of the Arg379–Ala380 bond in the C-terminal region of the vitronectin (Chain et al., 1991). The resulting disulfide-linked, two-chain (65 and 10 kDa) isoform of vitronectin is a conformationally distinct form of the glycoprotein that has been shown to preferentially bind heparin (Sane et al., 1990). However, the addition of high levels of exogenous heparin or pretreatment of Y. pestis with heparin had no effect on the interaction of Ail and vitronectin (data not shown). The processing of vitronectin by Pla (Fig. 3a, b) resulted in progressive degradation of the 75 kDa form of vitronectin and generated several degradation products similar in size to the 65 kDa form of vitronectin (Fig. 3b). However, preferential binding to the 65 kDa form of vitronectin was also observed with Pla– strains (Figs 1a and 2a, b), suggesting that there are likely multiple mechanisms that lead to preferential binding of the 65 kDa isoform of vitronectin to Ail. Finally, in several experiments where purified vitronectin was used in conjunction with Pla– Y. pestis strains (Figs 1b and 3d), no preferential binding to the 65 kDa form of vitronectin was observed.
Heat treatment of NHS or purified vitronectin at 56 °C for 30 min prevented recognition of vitronectin by Ail (Figs 1a and 2b). This was unexpected as previous studies had reported that heat inactivation of NHS or heat treatment (56 °C for 30 min) of purified vitronectin increased binding of vitronectin to N. meningitidis Opc and Msf (meningococcal surface fibril) (Griffiths et al., 2011; Sa E Cunha et al., 2010). Heating of vitronectin has been shown to modify its native structure by increasing the amount of a partially unfolded form termed ‘activated vitronectin’. Opc and Msf are reported to specifically recognize and bind activated vitronectin to mediate complement resistance in N. meningitidis. The present study, however, suggests that Y. pestis Ail is unlikely to recognize the same form of vitronectin recognized by Opc and Msf as heat treatment of vitronectin decreased, not increased, binding to Ail. It has also been reported that proteolytic processing of the vitronectin C terminus that forms the 65 kDa fragment also results in a partial unfolding of vitronectin (Sane et al., 1990). Thus, a partial unfolding of serum vitronectin, distinct from the partial unfolding associated with heat treatment, may be involved in its recognition by Ail.
Y. pestis Pla is a plasmid pPCP1-encoded outer membrane protein with aspartic protease activity (Sodeinde et al., 1992; Vandeputte-Rutten et al., 2001). Pla activates plasminogen to the serine protease plasmin, which subsequently cleaves fibrin. Pla also inactivates α2-antiplasmin and PAI-1. Together, these activities enhance fibrinolysis, which promotes Y. pestis survival and dissemination in its host (Sebbane et al., 2006; Sodeinde et al., 1992). PAI-1 is a serine protease that functions as the primary inhibitor of host plasminogen activators (Wiman, 1996). It exists in both active and inactive forms. The active form of PAI-1 binds to vitronectin, thus establishing a stable active reservoir of PAI-1 (Wiman et al., 1988). A previous study demonstrated that Y. pestis Pla and Salmonella enterica PgtE both degrade the vitronectin/PAI-1 complex (Haiko et al., 2010), facilitating fibrinolysis. Here, we show that the presence of Ail greatly facilitates the targeting and degradation of vitronectin, indicating that Ail may function hand-in-hand with Pla to rapidly target vitronectin likely facilitating the inactivation of PAI-1. The partial degradation of vitronectin and the binding of the cleaved vitronectin peptides may also facilitate or disrupt other vitronectin-dependent activities. Further studies will be required to determine the function, if any, of the degraded and bound fragments of vitronectin.
The finding that Y. pestis Ail binds C4BP, the primary fluid-phase regulator of the classical and lectin pathways, in addition to vitronectin is consistent with previous findings (Ho et al., 2014; Ngampasutadol et al., 2005). Importantly, many other pathogens bind both C4BP and vitronectin (Blom et al., 2009), suggesting that there may be a distinct advantage to recruiting both inhibitors. Indeed, the M. catarrhalis UspA2 protein has also been shown to bind both C4BP and vitronectin (Attia et al., 2006). In this case, the binding of vitronectin and not C4BP appeared to correlate with complement resistance. Overall, the ability to interfere with both the early and late stages of the complement cascade likely provides a more complete resistance to complement-mediated attacks. Further studies will be required to sort out the roles of C4BP, vitronectin and other serum factors in the pathogenesis of Y. pestis.
Acknowledgements
This research was supported by a grant from the National Institutes of Health (GM 100265). We thank Dr Anna Blom (Lund University, Malmö, Sweden) for providing anti-C4BP mAb 104 and Dr Jon Goguen (University of Massachusetts) for providing strain Yp JG150 (KIM5).
Abbreviations:
- C4BP
C4b-binding protein
- ECM
extracellular matrix
- HBS
heparin-binding site
- HIS
heat-inactivated sera
- MAC
membrane attack complex
- NHS
normal human sera
- PAI
plasminogen activator inhibitor
- T3SS
type III secretion system
References
- Attia A.S., Ram S., Rice P.A., Hansen E.J. (2006). Binding of vitronectin by the Moraxella catarrhalis UspA2 protein interferes with late stages of the complement cascade Infect Immun 74 1597–1611 10.1128/IAI.74.3.1597-1611.2006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra S.S., Jackson M.W., Ross J.A., Plano G.V. (2006). Calcium-regulated type III secretion of Yop proteins by an Escherichia coli hha mutant carrying a Yersinia pestis pCD1 virulence plasmid Infect Immun 74 1381–1386 10.1128/IAI.74.2.1381-1386.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra S.S., Styer K.L., O'Bryant D.M., Nilles M.L., Hinnebusch B.J., Aballay A., Plano G.V. (2008). Resistance of Yersinia pestis to complement-dependent killing is mediated by the Ail outer membrane protein Infect Immun 76 612–622 10.1128/IAI.01125-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard S., Fleury C., Su Y.C., Zipfel P.F., Koske I., Nordström T., Riesbeck K. (2014). Outer membrane protein OlpA contributes to Moraxella catarrhalis serum resistance via interaction with factor H and the alternative pathway J Infect Dis 210 1306–1310 10.1093/infdis/jiu241 . [DOI] [PubMed] [Google Scholar]
- Blom A.M., Hallström T., Riesbeck K. (2009). Complement evasion strategies of pathogens-acquisition of inhibitors and beyond Mol Immunol 46 2808–2817 10.1016/j.molimm.2009.04.025 . [DOI] [PubMed] [Google Scholar]
- Cambau E., Bordon F., Collatz E., Gutmann L. (1993). Novel gyrA point mutation in a strain of Escherichia coli resistant to fluoroquinolones but not to nalidixic acid Antimicrob Agents Chemother 37 1247–1252 10.1128/AAC.37.6.1247 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chain D., Korc-Grodzicki B., Kreizman T., Shaltiel S. (1991). Endogenous cleavage of the Arg-379-Ala-380 bond in vitronectin results in a distinct conformational change which ‘buries’ Ser-378, its site of phosphorylation by protein kinase A Biochem J 274 387–394 10.1042/bj2740387 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Fujimoto L.M., Yao Y., Plano G.V., Marassi F.M. (2015). Influence of the lipid membrane environment on structure and activity of the outer membrane protein Ail from Yersinia pestis Biochim Biophys Acta 1848 712–720 10.1016/j.bbamem.2014.11.021 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duensing T.D., van Putten J.P. (1997). Vitronectin mediates internalization of Neisseria gonorrhoeae by Chinese hamster ovary cells Infect Immun 65 964–970 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duensing T.D., Wing J.S., van Putten J.P. (1999). Sulfated polysaccharide-directed recruitment of mammalian host proteins: a novel strategy in microbial pathogenesis Infect Immun 67 4463–4468 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felek S., Krukonis E.S. (2009). The Yersinia pestis Ail protein mediates binding and Yop delivery to host cells required for plague virulence Infect Immun 77 825–836 10.1128/IAI.00913-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felek S., Tsang T.M., Krukonis E.S. (2010). Three Yersinia pestis adhesins facilitate Yop delivery to eukaryotic cells and contribute to plague virulence Infect Immun 78 4134–4150 10.1128/IAI.00167-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferracci F., Day J.B., Ezelle H.J., Plano G.V. (2004). Expression of a functional secreted YopN-TyeA hybrid protein in Yersinia pestis is the result of a+1 translational frameshift event J Bacteriol 186 5160–5166 10.1128/JB.186.15.5160-5166.2004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg A., Rosqvist R., Wolf-Watt H. (1994). Regulation and polarized transfer of the Yersinia outer proteins (Yops) involved in antiphagocytosis Trends Microbiol 2 14–19 10.1016/0966-842X(94)90339-5 . [DOI] [PubMed] [Google Scholar]
- Griffiths N.J., Hill D.J., Borodina E., Sessions R.B., Devos N.I., Feron C.M., Poolman J.T., Virji M. (2011). Meningococcal surface fibril (Msf) binds to activated vitronectin and inhibits the terminal complement pathway to increase serum resistance Mol Microbiol 82 1129–1149 10.1111/j.1365-2958.2011.07876.x . [DOI] [PubMed] [Google Scholar]
- Grosdent N., Maridonneau-Parini I., Sory M.P., Cornelis G.R. (2002). Role of Yops and adhesins in resistance of Yersinia enterocolitica to phagocytosis Infect Immun 70 4165–4176 10.1128/IAI.70.8.4165-4176.2002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiko J., Laakkonen L., Juuti K., Kalkkinen N., Korhonen T.K. (2010). The omptins of Yersinia pestis and Salmonella enterica cleave the reactive center loop of plasminogen activator inhibitor 1 J Bacteriol 192 4553–4561 10.1128/JB.00458-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallström T., Trajkovska E., Forsgren A., Riesbeck K. (2006). Haemophilus influenzae surface fibrils contribute to serum resistance by interacting with vitronectin J Immunol 177 430–436 10.4049/jimmunol.177.1.430 . [DOI] [PubMed] [Google Scholar]
- Hallström T., Jarva H., Riesbeck K., Blom A.M. (2007). Interaction with C4b-binding protein contributes to nontypeable Haemophilus influenzae serum resistance J Immunol 178 6359–6366 10.4049/jimmunol.178.10.6359 . [DOI] [PubMed] [Google Scholar]
- Hallström T., Blom A.M., Zipfel P.F., Riesbeck K. (2009). Nontypeable Haemophilus influenzae protein E binds vitronectin and is important for serum resistance J Immunol 183 2593–2601 10.4049/jimmunol.0803226 . [DOI] [PubMed] [Google Scholar]
- Hallström T., Singh B., Resman F., Blom A.M., Mörgelin M., Riesbeck K. (2011). Haemophilus influenzae protein E binds to the extracellular matrix by concurrently interacting with laminin and vitronectin J Infect Dis 204 1065–1074 10.1093/infdis/jir459 . [DOI] [PubMed] [Google Scholar]
- Härdig Y., Hillarp A., Dahlbäck B. (1997). The amino-terminal module of the C4b-binding protein alpha-chain is crucial for C4b binding and factor I-cofactor function Biochem J 323 469–475 10.1042/bj3230469 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch B.J., Jarrett C.O., Callison J.A., Gardner D., Buchanan S.K., Plano G.V. (2011). Role of the Yersinia pestis Ail protein in preventing a protective polymorphonuclear leukocyte response during bubonic plague Infect Immun 79 4984–4989 10.1128/IAI.05307-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D.K., Skurnik M., Blom A.M., Meri S. (2014). Yersinia pestis Ail recruitment of C4b-binding protein leads to factor I-mediated inactivation of covalently and noncovalently bound C4b Eur J Immunol 44 742–751 10.1002/eji.201343552. [DOI] [PubMed] [Google Scholar]
- Kolodziejek A.M., Sinclair D.J., Seo K.S., Schnider D.R., Deobald C.F., Rohde H.N., Viall A.K., Minnich S.S., Hovde C.J., other authors (2007). Phenotypic characterization of OmpX, an Ail homologue of Yersinia pestis KIM Microbiology 153 2941–2951 10.1099/mic.0.2006/005694-0 . [DOI] [PubMed] [Google Scholar]
- Kolodziejek A.M., Schnider D.R., Rohde H.N., Wojtowicz A.J., Bohach G.A., Minnich S.A., Hovde C.J. (2010). Outer membrane protein X (Ail) contributes to Yersinia pestis virulence in pneumonic plague and its activity is dependent on the lipopolysaccharide core length Infect Immun 78 5233–5243 10.1128/IAI.00783-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis L.A., Ram S. (2014). Meningococcal disease and the complement system Virulence 5 98–126 10.4161/viru.26515 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindler L.E., Tall B.D. (1993). Yersinia pestis pH 6 antigen forms fimbriae and is induced by intracellular association with macrophages Mol Microbiol 8 311–324 10.1111/j.1365-2958.1993.tb01575.x . [DOI] [PubMed] [Google Scholar]
- Lorange E.A., Race B.L., Sebbane F., Hinnebusch B.J. (2005). Poor vector competence of fleas and the evolution of hypervirulence in Yersinia pestis J Infect Dis 191 1907–1912 10.1086/429931 . [DOI] [PubMed] [Google Scholar]
- Lössner D., Abou-Ajram C., Benge A., Aumercier M., Schmitt M., Reuning U. (2009). Integrin alphavbeta3 upregulates integrin-linked kinase expression in human ovarian cancer cells via enhancement of ILK gene transcription J Cell Physiol 220 367–375 10.1002/jcp.21774 . [DOI] [PubMed] [Google Scholar]
- Marassi F.M., Ding Y., Schwieters C.D., Tian Y., Yao Y. (2015). Backbone structure of Yersinia pestis Ail determined in micelles by NMR-restrained simulated annealing with implicit membrane solvation J Biomol NMR 63 59–65 10.1007/s10858-015-9963-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milis L., Morris C.A., Sheehan M.C., Charlesworth J.A., Pussell B.A. (1993). Vitronectin-mediated inhibition of complement: evidence for different binding sites for C5b-7 and C9 Clin Exp Immunol 92 114–119 10.1111/j.1365-2249.1993.tb05956.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V.L., Bliska J.B., Falkow S. (1990). Nucleotide sequence of the Yersinia enterocolitica ail gene and characterization of the Ail protein product J Bacteriol 172 1062–1069 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V.L., Beer K.B., Heusipp G., Young B.M., Wachtel M.R. (2001). Identification of regions of Ail required for the invasion and serum resistance phenotypes Mol Microbiol 41 1053–1062 10.1046/j.1365-2958.2001.02575.x . [DOI] [PubMed] [Google Scholar]
- Nath A., Atkins W.M., Sligar S.G. (2007). Applications of phospholipid bilayer nanodiscs in the study of membranes and membrane proteins Biochemistry 46 2059–2069 10.1021/bi602371n . [DOI] [PubMed] [Google Scholar]
- Ngampasutadol J., Ram S., Blom A.M., Jarva H., Jerse A.E., Lien E., Goguen J., Gulati S., Rice P.A. (2005). Human C4b-binding protein selectively interacts with Neisseria gonorrhoeae and results in species-specific infection Proc Natl Acad Sci U S A 102 17142–17147 10.1073/pnas.0506471102 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström T., Blom A.M., Forsgren A., Riesbeck K. (2004). The emerging pathogen Moraxella catarrhalis interacts with complement inhibitor C4b binding protein through ubiquitous surface proteins A1 and A2 J Immunol 173 4598–4606 10.4049/jimmunol.173.7.4598 . [DOI] [PubMed] [Google Scholar]
- Perry R.D., Fetherston J.D. (1997). Yersinia pestis – etiologic agent of plague Clin Microbiol Rev 10 35–66 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putzker M., Sauer H., Sobe D. (2001). Plague and other human infections caused by Yersinia species Clin Lab 47 453–466 . [PubMed] [Google Scholar]
- Ram S., Mackinnon F.G., Gulati S., McQuillen D.P., Vogel U., Frosch M., Elkins C., Guttormsen H.K., Wetzler L.M., other authors (1999). The contrasting mechanisms of serum resistance of Neisseria gonorrhoeae and group B Neisseria meningitidis Mol Immunol 36 915–928 10.1016/S0161-5890(99)00114-5 . [DOI] [PubMed] [Google Scholar]
- Ram S., Cullinane M., Blom A.M., Gulati S., McQuillen D.P., Monks B.G., O'Connell C., Boden R., Elkins C., other authors (2001). Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae J Exp Med 193 281–295 10.1084/jem.193.3.281 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie T.K., Grinkova Y.V., Bayburt T.H., Denisov I.G., Zolnerciks J.K., Atkins W.M., Sligar S.G. (2009). Reconstitution of membrane proteins in phospholipid bilayer nanodiscs Methods Enzymol 464 211–231 10.1016/S0076-6879(09)64011-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosqvist R., Skurnik M., Wolf-Watz H. (1988). Increased virulence of Yersinia pseudotuberculosis by two independent mutations Nature 334 522–524 10.1038/334522a0 . [DOI] [PubMed] [Google Scholar]
- Sa E Cunha C., Griffiths N.J., Virji M. (2010). Neisseria meningitidis Opc invasin binds to the sulphated tyrosines of activated vitronectin to attach to and invade human brain endothelial cells PLoS Pathog 6 e1000911 10.1371/journal.ppat.1000911 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen E.M., Vaheri A., Pöllänen J., Stephens R., Andreasen P., Mayer M., Danø K., Gailit J., Ruoslahti E. (1989). Interaction of plasminogen activator inhibitor (PAI-1) with vitronectin J Biol Chem 264 6339–6343 . [PubMed] [Google Scholar]
- Sane D.C., Moser T.L., Parker C.J., Seiffert D., Loskutoff D.J., Greenberg C.S. (1990). Highly sulfated glycosaminoglycans augment the cross-linking of vitronectin by guinea pig liver transglutaminase. Functional studies of the cross-linked vitronectin multimers J Biol Chem 265 3543–3548 . [PubMed] [Google Scholar]
- Sebbane F., Jarrett C.O., Gardner D., Long D., Hinnebusch B.J. (2006). Role of the Yersinia pestis plasminogen activator in the incidence of distinct septicemic and bubonic forms of flea-borne plague Proc Natl Acad Sci U S A 103 5526–5530 10.1073/pnas.0509544103 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet M., Riot B., Fortineau N., Berche P. (1996). Invasin production by Yersinia pestis is abolished by insertion of an IS200-like element within the inv gene Infect Immun 64 375–379 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B., Su Y.C., Riesbeck K. (2010). Vitronectin in bacterial pathogenesis: a host protein used in complement escape and cellular invasion Mol Microbiol 78 545–560 10.1111/j.1365-2958.2010.07373.x . [DOI] [PubMed] [Google Scholar]
- Skurnik M., Wolf-Watz H. (1989). Analysis of the yopA gene encoding the Yop1 virulence determinants of Yersinia spp Mol Microbiol 3 517–529 10.1111/j.1365-2958.1989.tb00198.x . [DOI] [PubMed] [Google Scholar]
- Sodeinde O.A., Subrahmanyam Y.V., Stark K., Quan T., Bao Y., Goguen J.D. (1992). A surface protease and the invasive character of plague Science 258 1004–1007 10.1126/science.1439793 . [DOI] [PubMed] [Google Scholar]
- Tsang T.M., Felek S., Krukonis E.S. (2010). Ail binding to fibronectin facilitates Yersinia pestis binding to host cells and Yop delivery Infect Immun 78 3358–3368 10.1128/IAI.00238-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Une T., Brubaker R.R. (1984). In vivo comparison of avirulent Vwa- and Pgm- or Pstr phenotypes of yersiniae Infect Immun 43 895–900 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeputte-Rutten L., Kramer R.A., Kroon J., Dekker N., Egmond M.R., Gros P. (2001). Crystal structure of the outer membrane protease OmpT from Escherichia coli suggests a novel catalytic site EMBO J 20 5033–5039 10.1093/emboj/20.18.5033 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viboud G.I., Bliska J.B. (2004). Yersinia outer proteins: role in modulation of host cell signalling responses and pathogenesis Annu Rev Microbiol 59 69–89 . [DOI] [PubMed] [Google Scholar]
- Vogt J., Schulz G.E. (1999). The structure of the outer membrane protein OmpX from Escherichia coli reveals possible mechanisms of virulence Structure 7 1301–1309 10.1016/S0969-2126(00)80063-5 . [DOI] [PubMed] [Google Scholar]
- Wachtel M.R., Miller V.L. (1995). In vitro and in vivo characterization of an ail mutant of Yersinia enterocolitica Infect Immun 63 2541–2548 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiman B. (1996). Plasminogen activator inhibitor 1 in thrombotic disease Curr Opin Hematol 3 372–378 10.1097/00062752-199603050-00007 . [DOI] [PubMed] [Google Scholar]
- Wiman B., Almquist A., Sigurdardottir O., Lindahl T. (1988). Plasminogen activator inhibitor 1 (PAI) is bound to vitronectin in plasma FEBS Lett 242 125–128 10.1016/0014-5793(88)80999-2 . [DOI] [PubMed] [Google Scholar]
- Yamashita S., Lukacik P., Barnard T.J., Noinaj N., Felek S., Tsang T.M., Krukonis E.S., Hinnebusch B.J., Buchanan S.K. (2011). Structural insights into Ail-mediated adhesion in Yersinia pestis Structure 19 1672–1682 10.1016/j.str.2011.08.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]