Animals that develop in shallow soils are susceptible to lethal temperatures during heat waves. We found that developing lizards from four populations entered cardiac arrest at temperatures above 46°C. Since temperatures of natural nests can presently exceed this limit, global warming would further reduce recruitment of young.
Keywords: Critical thermal maximum, embryo, heart rate, survival, temperature, thermal tolerance
Abstract
The frequency and magnitude of heat waves have increased in recent decades, imposing additional stresses on organisms in extreme environments. Most reptilian embryos are regularly exposed to thermal stress because they develop in shallow, warm soils for weeks to months. We studied cardiac performance during warming to infer lethal temperatures for embryonic lizards in the Sceloporus undulatus complex. Embryos from four populations throughout the geographical range (New Jersey, South Carolina, Colorado, and Arizona) were warmed at a rate observed in natural nests. Embryos from all populations exhibited a similar pattern of thermal sensitivity, as follows: heart rate rose between 34 and 41°C, remained stable between 41 and 44°C, and dropped sharply between 44 and 47°C. No embryos recovered from cardiac arrest, indicating that the upper lethal temperature was ≤47°C. Despite the putative selective pressures, the thermal limit to cardiac performance seems to have been conserved during the evolution of this species.
Introduction
In the last decade, researchers have increasingly used organismal tolerances to predict impacts of climate change on population dynamics or geographical distributions (Braune et al., 2008; Buckley et al., 2010; Kearney, 2011; Molnar et al., 2013). The development and application of their models depend on empirical estimates of tolerance during realistic changes in environmental conditions. Many, if not most, organisms experience rapid and wide fluctuations in temperature within hours to days. Indeed, heat waves have become common in recent decades (Hansen et al., 2012) and should occur even more frequently in the coming decades (IPCC, 2007). When the effects of such acute stresses were unknown, studies of chronic stresses were used to parameterize models (Crozier and Dwyer, 2006; Deutsch et al., 2008). This approach must bias predictions, because organisms thrive during periodic exposure to temperatures that prove lethal during chronic exposure (Siddiqui and Barlow, 1972, 1973; Siddiqui et al., 1973). Therefore, organismal responses to acute stresses must be documented to infer the biological impacts of climate change (e.g. see Huey et al., 2009).
Animals should be particularly vulnerable to sudden warming at the embryonic stage. Embryos, being relatively small and immobile, have limited capacities to thermoregulate (but see Du et al., 2011). Despite the danger that acute thermal stress should pose to embryos, we know virtually nothing about the impacts of this stress on embryonic performance. Studies of natural nests suggest that thermal fluctuations have little direct impact on embryonic performance (Cagle et al., 1993; Thompson et al., 1996; Shine et al., 1997); however, these studies cannot reveal the impacts of temperatures that exceed the current range. Although many researchers have manipulated fluctuations in temperature (Qualls and Shine, 1998; Ashmore and Janzen, 2003; Shine, 2004; Mullins and Janzen, 2006; Oufiero and Angilletta, 2006; Du and Shine, 2010; Warner et al., 2010; Warner and Shine, 2011), these experiments were not designed to stress embryos. Consequently, embryos were not exposed to temperatures that approached or exceeded their thermal limit of performance.
Admittedly, quantifying the impacts of thermal stress on embryos remains difficult because no standard assay exists. For other life stages, researchers can choose from several assays to gauge whether animals can tolerate severe and sudden thermal stress (see reviews by Lutterschmidt and Hutchison, 1997; Hoffmann et al., 2003; Angilletta, 2009). These assays focus on the maximal temperature at which an animal can move or respond to mechanical stimuli, usually referred to as the critical thermal maximum (Cowles and Bogert, 1944) or knockdown temperature (Huey et al., 1992). By warming an animal over the course of minutes to hours, a researcher can pinpoint the temperature at which the animal loses some ability. For example, isopods lost their ability to roll over at temperatures around 37°C (Castaneda et al., 2004), and flies lost their ability to fly between 35 and 42°C (Gilchrist and Huey, 1999). Likewise, various species of fish (Beitinger et al., 2000) and reptiles (Licht et al., 1966; Winne and Keck, 2005) ceased to move at specific temperatures. However, these assays target behaviours that one cannot observe directly for embryos.
Fortunately, methodological advances enable one to record heart rates of amniotic embryos without disturbing their growth and development (Lierz et al., 2006; Du and Shine, 2008). Changes in heart rate reveal physiological responses to warming. As an organism warms, its heart initially beats faster (Du et al., 2010c). This acceleration enhances the distribution of oxygen and nutrients to meet the greater metabolic demands of a warm body (Du et al., 2009, 2010b). But the beating of the heart cannot accelerate indefinitely. At some temperature, the heart will fail to beat faster or even fail to beat at all. Such changes in cardiac performance have been used to infer thermal limits in adult ectotherms (Stillman and Somero, 1996).
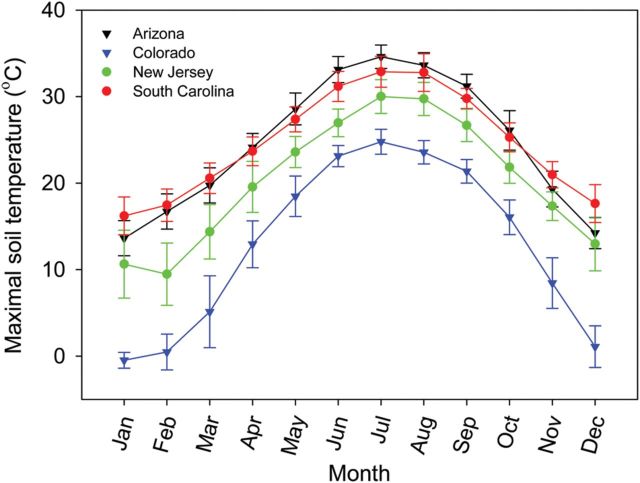
We used changes in heart rate to quantify the heat tolerance of embryonic lizards in the Sceloporus undulatus complex. This paraphyletic biological species comprises four major clades that collectively cover much of the USA (Leaché and Reeder, 2002; Leaché, 2009). Both morphology and life history vary within clades according to environmental temperatures (Angilletta and Sears, 2004; Angilletta et al., 2004, 2006). However, the heat tolerance of adults seems highly conserved throughout the range, possibly because of effective thermoregulation (Ehrenberger, 2010). As embryos cannot behaviourally thermoregulate to the same degree that adults can, we expected their heat tolerances to reflect local climates (Fig. 1); specifically, we expected embryos from warm, southern regions to tolerate higher temperatures than do embryos from cool, northern regions. To test this prediction, we compared the thermal sensitivity and critical thermal maximum of cardiac performance between northern and southern populations from two clades.
Figure 1:
Soils in Arizona and South Carolina are warmer than those in Colorado and New Jersey. Maximal soil temperatures at a depth of 5 cm at localities closest to our populations: AZ, 33.2° N, 110.0° W; NJ, 39.8° N, 74.7° W; SC, 33.6° N, 81.9° W; and CO, 37.0° N, 105.6° W. Each datum is the mean and standard deviation of monthly maxima between 1979 and 2010, averaged over a grid of 0.312° × 0.312° (National Center for Atmospheric Research, Boulder, CO, USA).
Materials and methods
Ethics statement
This study was conducted according to recommendations in the Guidelines for Use of Live Amphibians and Reptiles in Field and Laboratory Research of the American Society of Ichthyologists and Herpetologists. All procedures were approved by the Animal Care and Use Committee of Arizona State University (protocol no. 11-1161).
Collection and husbandry
Although lizards throughout the range of S. undulatus reproduce at different frequencies, lizards in all populations produce eggs between May and July. Therefore, we collected gravid females in May 2012. These lizards came from the following four localities: Atlantic County, NJ; Edgefield County, SC; Costilla County, CO; and Gila County, AZ. Lizards were transported to Arizona State University, where they were housed in plastic terraria with a substrate of moist sand. Terraria were kept in a controlled environment, with a 12 h light–12 h dark cycle, and an ambient temperature of 33°C during photophase and 20°C during scotophase. Water was available at all times, and crickets were provided daily.
Acquisition and care of eggs
Females either laid eggs naturally or were induced with an injection of oxytocin, as described by Storm and Angilletta (2007). Freshly laid eggs were weighed and placed in plastic containers for incubation. Each container contained ∼400 g of sand mixed with 4 g of water, resulting in a water potential of −10 kPa (Oufiero and Angilletta, 2006).
All containers were kept in a programmable incubator (model DR-36VL; Percival Scientific). The incubator maintained a daily cycle of temperature, ranging from 20.4 to 34.7°C, and a relative humidity of 85%. The thermal cycle was based on the temperatures of nests constructed by females in artificial thermal gradients and natural environments (Warner and Andrews, 2002; Angilletta et al., 2009). Water that evaporated from the containers was replaced weekly. Although this procedure permitted small changes in water potential, eggs incubated in these conditions absorb water at a relatively constant rate throughout incubation (Angilletta et al., 2000). One egg from each population was incubated in each container to prevent covariation between environmental and genetic factors.
Thermal sensitivity of heart rate
We measured the effect of temperature on the heart rates of embryos throughout development. Heart rates were measured using a commercially available system of infrared sensors (Buddy Egg Monitor; Avitronics, Truro, UK); heart rates estimated by this method compare favourably with those estimated by other methods (Lierz et al., 2006). Heart rates of lizard embryos became detectable about a week after oviposition. From this point onwards, we recorded heart rates at 24 and 34°C for one embryo of each clutch (23, 17, 7, and 7 clutches from NJ, SC, CO, and AZ, respectively). Measurements were made weekly, at the point in the daily cycle when eggs would normally experience these temperatures (10.00 and 14.00 h for 24 and 34°C, respectively). Immediately before each measurement, eggs were temporarily moved to containers of moist sand, similar to the incubation containers. Containers were placed in an incubator set at either 24 or 34°C. Eggs were removed from the incubator one at a time, enabling us to transfer each egg quickly between the incubator and the heart rate monitor. Heart rate was recorded within 30 s to minimize the cooling of embryos during measurements. After measurements at each temperature, the eggs were returned to their incubation containers and placed in the incubator that maintained their daily cycle of temperature.
Using an information-theoretical approach (Burnham and Anderson, 2002), we identified the best statistical model of heart rate. General linear modelling was used to estimate the effects of temperature, initial egg mass, stage of incubation (estimated as days since oviposition), and population. To avoid pseudoreplication, we also included the identity of each egg as a random factor (Zuur et al., 2009). Initially, we modelled all main effects and interactions. Then, following Crawley (2007), we dropped terms from the maximal model and used Akaike's information criterion to confirm the improved fit of the simplified model. We removed terms from the highest order to the lowest order until the model with the lowest Akaike's information criterion was obtained. All models were fitted using the nlme library (Pinheiro et al., 2013) of the R Statistical Package (R Development Core Team, 2011).
Thermal limits of heart rate
We estimated the thermal limit of cardiac performance by monitoring the heart rates of embryos during continuous warming. Measurements began at 14.00 h when eggs would normally experience 34°C (see ‘Acquisition and care of eggs’). An egg from each clutch was randomly assigned to a warming group and a control group (19, 11, 5, and 7 clutches from NJ, SC, CO, and AZ, respectively). Eggs of the former group were warmed by 3°C h−1 from 34°C to their upper lethal limits, whereas eggs of the latter group were handled in a similar manner while remaining at their usual cycle of temperature. The rate of warming lies within the range of rates reported for natural nests (Angilletta et al., 2009). To control warming while recording heart rates at specific intervals, we warmed only two eggs per trial. The two control eggs in each trial were from the same clutches.
We took precautions to ensure that eggs warmed precisely and remained at the target temperatures during measurements of heart rate. At the start of each trial, eggs were weighed to the nearest 0.01 mg. Each egg was positioned in a padded dish and sealed in a glass jar (125 ml); the jar contained ∼1 ml of distilled water to prevent the egg from desiccating through evaporation. A thermocouple was inserted through the lid of the jar to monitor the air temperature inside. The jar was then submerged in a water bath, whose temperature was regulated by a programmable circulator (Proline 855C; LAUDA-Brinkmann, LP). Initially, the temperature of the bath was set to 34°C. A few minutes after the temperature inside the jar reached 34°C, the jar was transferred to a small container of water at the same temperature as the bath. This container was carried into an environmental chamber, which was set at the same temperature as the bath. Inside the chamber, the egg was quickly removed from the jar and placed inside a heart-rate monitor. Although the heart rate was recorded manually, we also used a video camera to record the digital panel of the heart-rate monitor for ∼30 s; this recording enabled us to confirm any values that seemed unusual during data analysis. Immediately after this recording, the egg was placed in its jar and returned to the water bath. The temperature of the bath was incremented by 0.5°C, and the entire procedure was repeated. For each egg, the trial ended when we were unable to obtain a heart rate for two consecutive intervals. Eggs in the control group remained at their usual cycle of temperature; each time that heart rates of the warming embryos were measured, the control eggs were placed in a small box for the same duration to simulate the handling that occurs during measurements of heart rate. At the end of the trial, eggs were reweighed to estimate water loss during the experiment.
We used general additive mixed modelling to compare thermal sensitivities of heart rate among populations. An additive model permitted a non-linear response to temperature, without requiring us to specify a function (Zuur et al., 2009). Consequently, we preferred this approach to one that assumed an exponential, an asymptotic, or a piecewise function (e.g. Arrhenius breakpoints). Potential explanatory variables included temperature, population, and stage of incubation. To avoid pseudoreplication, the identity of each embryo was included as a random factor. For each combination of factors, we fitted a model with a fixed error term and a model in which the error in heart rate increased with increasing temperature.
Given that non-linear models were unable to fit the sharp drop in heart rate at high temperatures, we used the raw data to estimate the critical thermal maximum of heart rate, i.e. the temperature at which heart rate dropped to zero. The method of general least squares was used to model the critical thermal maximum as a function of population, egg mass, and stage of incubation. A separate error term was estimated for each population, because the variances appeared to differ. Analyses were performed with the mgcv (Wood, 2004) and nlme (Pinheiro et al., 2013) libraries of R. The most likely model was selected on the basis of Akaike's information criterion.
Phenotypes of hatchlings
After 45 days of incubation, eggs were checked daily for signs of hatching. Hatchlings were weighed to the nearest 0.01 g and measured to the nearest millimetre (snout-to-vent length). Descriptive statistics are reported as means ± 95% confidence interval.
Results
Thermal sensitivity of cardiac performance
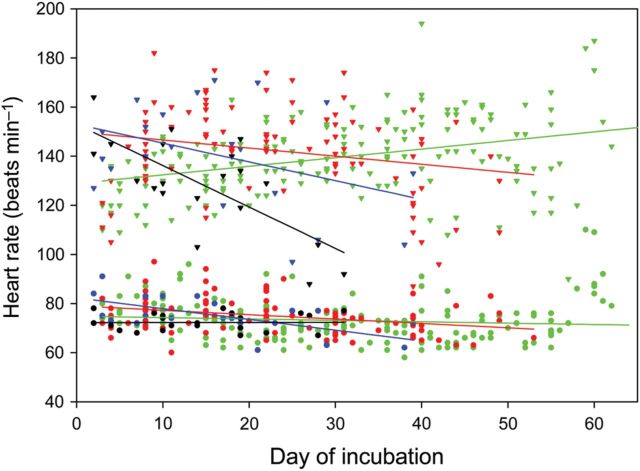
The most likely model of heart rate included many sources of variation, including egg mass at oviposition, population of origin, stage of incubation, and some interactions among these variables (Table 1). Nevertheless, body temperature affected the heart rate of embryos far more than any other factor. At all stages of incubation, heart rate at 34°C exceeded that at 24°C by at least 70%. For most populations, mean heart rate remained stable or decreased slightly throughout incubation (Fig. 2).
Table 1:
Inferential statistics for the most likely model of heart rate at tolerable temperatures
| Effect | Effect d.f. | Error d.f. | F | P-value |
|---|---|---|---|---|
| Intercept | 1 | 609 | 242.93 | <0.0001 |
| Mass at oviposition (g) | 1 | 609 | 0.68 | 0.4096 |
| Stage of incubation (days) | 1 | 609 | 5.91 | 0.0153 |
| Population (AZ, CO, NJ, SC) | 3 | 609 | 3.75 | 0.0109 |
| Temperature (24 or 34°C) | 1 | 609 | 341.36 | <0.0001 |
| Mass × stage | 1 | 609 | 4.80 | 0.0289 |
| Stage × population | 3 | 609 | 2.47 | 0.0607 |
| Stage × temperature | 1 | 609 | 22.14 | <0.0001 |
| Population × temperature | 3 | 609 | 8.03 | <0.0001 |
| Stage × population × temperature | 3 | 609 | 14.58 | <0.0001 |
The residual error was modelled with separate terms for each population and each temperature. The standard deviation of the mean for embryos from Colorado, New Jersey, and South Carolina was estimated to be 1.5, 1.4, and 1.4 times greater, respectively, than that for embryos from Arizona. The standard deviation at 34°C was 2.2 times greater than that at 24°C.
Figure 2:
Mean heart rates of embryos at 24 (circles) and 34°C (triangles) changed slightly throughout development. Data are for lizards from Arizona (black symbols), Colorado (blue symbols), New Jersey (green symbols), and South Carolina (red symbols). Lines represent the relationships estimated from the most likely statistical model.
Thermal limit of cardiac performance
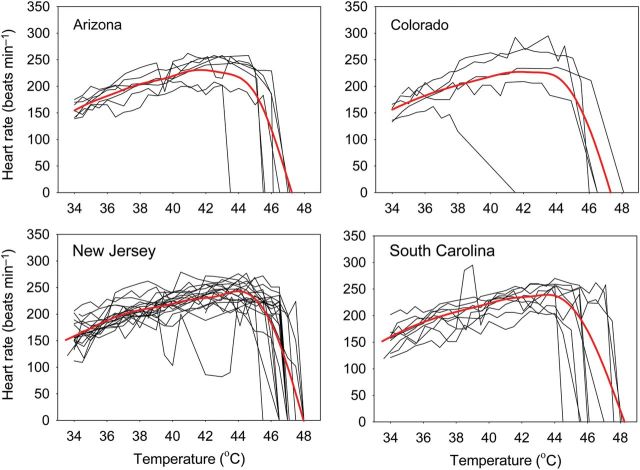
When embryos were warmed continuously, cardiac performance changed non-linearly in a manner commonly observed for physiological performances (Fig. 3). Warming from 34 to 41°C caused hearts to beat faster. Further warming caused heart rates to stabilize initially and then to drop precipitously. Cardiac arrest occurred at body temperatures ranging from to 41.5 to 48.1°C, but >93% of embryos succumbed at body temperatures >45°C. Older embryos in larger eggs reached slightly higher temperatures before cardiac arrest (Table 2). Although embryos from eastern populations reached higher heart rates (see Fig. 3), embryos from all populations underwent cardiac arrest at a similar median temperature, i.e. 46.5, 46.5, 47.0, and 46.5°C for embryos from Arizona, Colorado, New Jersey, and South Carolina, respectively (Table 3). The survival of heated eggs was extremely poor (7% hatching success) compared with that of eggs in the control group (51% hatching success). Survival was not influenced by water loss during warming, because the masses of eggs did not change appreciably during the warming experiment; mean masses before and after warming were 1.47 and 1.48 g, respectively (paired t = − 1.83, d.f. = 27, P = 0.08).
Figure 3:
Heart rate increased during warming until embryos reached 41–44°C and dropped sharply between 44 and 47°C. Each black line depicts the trajectory of hearts rate for a single embryo. The red lines depict relationships estimated from the most likely general additive mixed model.
Table 2:
Inferential statistics for the most likely model of heart rate during continuous warming
| Effect | d.f. | F | P-value |
|---|---|---|---|
| f(T) for embryos from Arizona | 7.79 | 42.73 | <0.0001 |
| Deviation from f(T) for embryos from Colorado | 2.00 | 0.001 | 0.9993 |
| Deviation from f(T) for embryos from New Jersey | 5.82 | 6.47 | <0.0001 |
| Deviation from f(T) for embryos from South Carolina | 4.60 | 5.10 | 0.0002 |
The generalized additive model describes heart rate as a function of temperature, or f(T), instead of using a fixed parameter to describe the effect of temperature. Additional functions were included to describe how heart rates of embryos from each population deviated from those of embryos from Arizona.
Table 3:
Inferential statistics for the most likely model of the critical thermal maximum of heart rate
| Effect | Effect d.f. | Error d.f. | F | P-value |
|---|---|---|---|---|
| Mass at oviposition (g) | 1 | 34 | 17.55 | 0.1074 |
| Stage of incubation (days) | 1 | 34 | 11.97 | 0.8055 |
| Population of origin (AZ, CO, NJ, SC) | 3 | 34 | 0.54 | 0.0936 |
| Mass × stage | 1 | 34 | 9.96 | 0.0033 |
Discussion
Heart rates of embryonic lizards either remained stable or decreased slightly during development. Generally, any developmental effect on heart rate was very small relative to the acute effect of temperature. The only pronounced decrease in heart rate during development occurred within embryos from Arizona; nevertheless, this pattern, which was observed only at 34°C, might have resulted from poor sampling of heart rates during later stages combined with the high variance of heart rate at 34°C (see Fig. 2). Our findings are in agreement with those of the only other study of developmental changes in heart rates of embryonic reptiles. In that study, heart rates of turtles at high temperature decreased during development, but heart rates at low temperature remained stable during development (Birchard and Reiber, 1996). In other embryonic vertebrates, heart rates increased, decreased, or remained stable during development (Tazawa, 2005; Brown et al., 2012). Although heart rate often remains stable, the growth of embryos accelerates throughout most of development (Vleck and Vleck, 1980; Vleck and Hoyt, 1991). Consequently, the cardiac output needed for embryonic performance must come from an exponential increase of cardiac muscle, leading to greater stroke volume over time. The exponential increase in the stroke volume during the development of avian embryos accords with this interpretation (Tazawa, 2005).
Thermal sensitivities of embryonic heart rate in S. undulatus were similar to those of other species (Benfey and Bennett, 2009; Du and Shine, 2010; Lee and Lee, 2010). Du and colleagues (2010b, c) compared thermal sensitivities among several species of reptiles, including lizards, snakes, and turtles; heart rates increased by a factor of 1.5–2.6 with a warming of 10°C. In contrast, heart rates of avian embryos increase by 2- to 3-fold when warmed to the same extent (Nechaeva, 2011). In S. undulatus, heart rate barely increased by 2-fold over the range of 20–34°C (Du et al., 2010a). Our measurements confirmed the low thermal sensitivity of reptilian heart rate, and also established that this thermal sensitivity remains stable throughout development (see Fig. 2). As with other physiological performances (Huey and Stevenson, 1979; Angilletta et al., 2002b), the thermal sensitivity of heart rate depends on the range of temperatures; during continuous warming, heart rate initially increased rapidly but ultimately reached a maximum, resulting in a non-linear response to temperature over a broad thermal range (see Fig. 3).
Unlike previous experiments, we applied thermal stress until embryos failed to sustain cardiac performance. Heart rate decelerated rapidly at temperatures between 44 and 47°C (see Fig. 3). Contrary to our prediction, we did not find that the critical thermal maximum of cardiac performance was greater for embryos from warmer regions. This conservation of heat tolerance among populations suggests several hypotheses. First, selection caused by infrequent but extreme warming in all populations might have driven the critical thermal maximum to the highest possible level. If so, future warming would negatively impact the growth of populations without opportunities for adaptation to restore the mean fitness. Alternatively, mothers in different regions might choose nesting sites that never exceed the critical thermal maximum. In New Jersey, females construct nests at shallow depths (∼6 cm) in the most exposed soils they can find (Angilletta et al., 2009). In more southerly environments, females can lay their eggs in deeper or shadier sites to compensate for the greater solar radiation. For nesting behaviour to compensate for changes in climate, females must differ genetically in their propensity to choose particular nesting sites (McGaugh et al., 2010). Although nesting behaviour can compensate for temporal or spatial variation in environmental temperatures (Doody et al., 2006; Telemeco et al., 2009; Refsnider and Janzen, 2012), we currently know little about variation in the nesting behaviour of S. undulatus (Warner and Andrews, 2002; Angilletta et al., 2009). Still, of the two hypotheses, the first one seems more plausible because nest temperatures in one of the coldest portions of the range sometimes exceed the critical thermal maximum (see below). Also, if adaptive nesting behaviour were a major factor, relaxed selection at the embryonic stage would enable critical thermal maxima to diverge among populations by genetic drift.
Interestingly, the temperatures that caused cardiac arrest in embryonic lizards exceeded temperatures that immobilized adult lizards from the same populations. Depending on its geographical origin, an adult cannot roll over at temperatures above 41–44°C (Angilletta et al., 2002a; Ehrenberger, 2010). Unfortunately, we do not know the critical thermal maximum of heart rate in adults. In previous studies of S. undulatus (Dzialowski and O'Connor, 2001) and its congener S. occidentalis (Francis and Brooks, 1970), heart rates of adults increased linearly during warming, but warming did not exceed 35°C. In more distantly related species, heart rates of adult lizards increased linearly or almost linearly during warming to 40°C (Licht, 1965; Arad, 1995). Logically, the thermal limit of cardiac performance (and survival) must equal or exceed the thermal limit of locomotor performance. Nevertheless, the large difference between the thermal limits of embryonic heart rate and adult righting response suggest that embryos tolerate higher temperatures than adults do. Given that we pushed embryos to the point of cardiac arrest, we were unable to quantify any impact of sub-lethal stresses. In other words, exposure to temperatures slightly below the lethal limit might have impacted the phenotype or survival of embryos later in development.
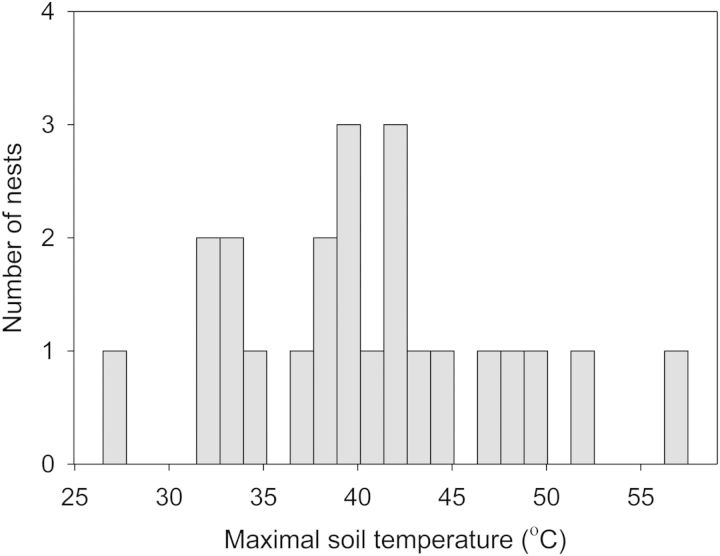
Studies of acute thermal stress provide insights needed to model the biological impacts of global warming. Having defined the temperatures that cause cardiac arrest, we can now infer whether temperatures in natural nests will remain within tolerable limits. In shallow soils, where lizards lay their eggs, brief exposures to extreme temperatures occur daily (Shine et al., 2003; Angilletta et al., 2009). For example, 22% of a sample of nests in New Jersey exceeded the critical thermal maximum for cardiac performance at least once (Fig. 4). This frequency of lethal events will increase if anthropogenic factors continue to drive global warming. To make matters worse, mortality can result not only from a single brief exposure to a high temperature but also from multiple exposures to lower temperatures. Thus, researchers should begin to consider the cumulative damage that occurs from sublethal heat stress. Surprisingly, we know even less about the sudden and cumulative impacts of extreme temperatures on embryos than we know about the ways in which embryos and their mothers thermoregulate (Du et al., 2011; Schwarzkopf and Andrews, 2012; Shine, 2012). This gap in our knowledge exists because early research focused on constant temperatures and later research focused on non-lethal effects of fluctuating temperatures (Overall, 1994; Qualls and Shine, 1998; Shine and Downes, 1999; Andrews et al., 2000; Oufiero and Angilletta, 2006). Clearly, this gap must be closed if we wish to know whether heat waves will threaten recruitment in populations.
Figure 4:
Embryos in natural nests experience temperatures that caused cardiac arrest in our experiment. The plot shows the distribution of maximal temperatures for nests in New Jersey (Angilletta et al., 2009); nearly 22% of the nests exceeded the critical thermal maximum for this population (median = 47°C).
Acknowledgements
We thank Jason Borchert and Travis Rusch for collecting lizards. Donovan Kilby and Davina Kumar helped to care for eggs and measure heart rates. Ofir Levy obtained data on soil temperatures. This work was supported by the National Science Foundation (grant number EF-1065638).
References
- 1.Andrews RM, Mathies T, Warner DA. (2000) Effect of incubation temperature on morphology, growth, and survival of juvenile Sceloporus undulatus. Herpetol Monogr 14: 420–431. [Google Scholar]
- 2.Angilletta MJ, Winters RS, Dunham AE. (2000) Thermal effects on the energetics of lizard embryos: Implications for hatchling phenotypes. Ecology 81: 2957–2968. [Google Scholar]
- 3.Angilletta MJ, Hill T, Robson MA. (2002a) Is physiological performance optimized by thermoregulatory behavior?: A case study of the eastern fence lizard, Sceloporus undulatus. J Therm Biol 27: 199–204. [Google Scholar]
- 4.Angilletta MJ, Niewiarowski PH, Navas CA. (2002b) The evolution of thermal physiology in ectotherms. J Therm Biol 27: 249–268. [Google Scholar]
- 5.Angilletta MJ, Niewiarowski PH, Dunham AE, Leache AD, Porter WP. (2004) Bergmann's clines in ectotherms: illustrating a life-history perspective with sceloporine lizards. Am Nat 164: E168–E183. [DOI] [PubMed] [Google Scholar]
- 6.Angilletta MJ, Sears MW. (2004) Body size clines in sceloporus lizards: proximate mechanisms and demographic constraints. Integr Comp Biol 44: 433–442. [DOI] [PubMed] [Google Scholar]
- 7.Angilletta MJ, Oufiero CE, Leache AD. (2006) Direct and indirect effects of environmental temperature on the evolution of reproductive strategies: an information-theoretic approach. Am Nat 168: E123–E135. [DOI] [PubMed] [Google Scholar]
- 8.Angilletta MJ. (2009) Thermal Adaptation: a Theoretical and Empirical Synthesis. Oxford University Press, Oxford. [Google Scholar]
- 9.Angilletta MJ, Sears MW, Pringle RM. (2009) Spatial dynamics of nesting behavior: lizards shift microhabitats to construct nests with beneficial thermal properties. Ecology 90: 2933–2939. [DOI] [PubMed] [Google Scholar]
- 10.Arad Z. (1995) Physiological responses to increasing ambient temperature in three ecologically different, congeneric lizards (Gekkoninae: Ptyodactylus). Comp Biochem Physiol A Physiol 112: 305–311. [Google Scholar]
- 11.Ashmore G, Janzen F. (2003) Phenotypic variation in smooth softshell turtles (Apalone mutica) from eggs incubated in constant versus fluctuating temperatures. Oecologia 134: 182–188. [DOI] [PubMed] [Google Scholar]
- 12.Beitinger TL, Bennett WA, McCauley RW. (2000) Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environ Biol Fish 58: 237–275. [Google Scholar]
- 13.Benfey TJ, Bennett LE. (2009) Effect of temperature on heart rate in diploid and triploid brook charr, Salvelinus fontinalis, embryos and larvae. Comp Biochem Physiol A Mol Integr Physiol 152: 203–206. [DOI] [PubMed] [Google Scholar]
- 14.Birchard G, Reiber C. (1996) Heart rate during development in the turtle embryo: effect of temperature. J Comp Physiol B 166: 461–466. [DOI] [PubMed] [Google Scholar]
- 15.Braune E, Richter O, Sondgerath D, Suhling F. (2008) Voltinism flexibility of a riverine dragonfly along thermal gradients. Glob Change Biol 14: 470–482. [Google Scholar]
- 16.Brown CA, Galvez F, Green CC. (2012) Embryonic development and metabolic costs in gulf killifish Fundulus grandis exposed to varying environmental salinities. Fish Physiol Biochem 38: 1071–1082. [DOI] [PubMed] [Google Scholar]
- 17.Buckley LB, Urban MC, Angilletta MJ, Crozier LG, Rissler LJ, Sears MW. (2010) Can mechanism inform species' distribution models? Ecol Lett 13: 1041–1054. [DOI] [PubMed] [Google Scholar]
- 18.Burnham KP, Anderson DR. (2002) Model Selection and Multimodel Inference: a Practical Information-Theoretic Approach. Springer, New York. [Google Scholar]
- 19.Cagle KD, Packard GC, Miller K, Packard MJ. (1993) Effects of the microclimate in natural nests on the development of embryonic painted turtles, Chrysemys picta. Funct Ecol 7: 653–660. [Google Scholar]
- 20.Castaneda LE, Lardies MA, Bozinovic F. (2004) Adaptive latitudinal shifts in the thermal physiology of a terrestrial isopod. Evol Ecol Res 6: 579–593. [Google Scholar]
- 21.Cowles RB, Bogert CM. (1944) A preliminary study of the thermal requirements of desert reptiles. Bull Am Mus Nat Hist 83: 263–296. [Google Scholar]
- 22.Crawley MJ. (2007) The R Book. John Wiley & Sons Ltd, Chichester. [Google Scholar]
- 23.Crozier L, Dwyer G. (2006) Combining population-dynamic and ecophysiological models to predict climate-induced insect range shifts. Am Nat 167: 853–866. [DOI] [PubMed] [Google Scholar]
- 24.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. (2008) Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA 105: 6669–6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doody JS, Guarino E, Georges A, Corey B, Murray G, Ewert M. (2006) Nest site choice compensates for climate effects on sex ratios in a lizard with environmental sex determination. Evol Ecol 20: 307–330. [Google Scholar]
- 26.Du W-G, Shine R. (2008) The influence of hydric environments during egg incubation on embryonic heart rates and offspring phenotypes in a scincid lizard (Lampropholis guichenoti). Comp Biochem Physiol A Mol Integr Physiol 151: 102–107. [DOI] [PubMed] [Google Scholar]
- 27.Du W-G, Radder RS, Sun B, Shine R. (2009) Determinants of incubation period: do reptilian embryos hatch after a fixed total number of heart beats? J Exp Biol 212: 1302–1306. [DOI] [PubMed] [Google Scholar]
- 28.Du W-G, Shine R. (2010) Why do the eggs of lizards (Bassiana duperreyi: Scincidae) hatch sooner if incubated at fluctuating rather than constant temperatures? Biol J Linn Soc 101: 642–650. [Google Scholar]
- 29.Du W-G, Warner Daniel A, Langkilde T, Robbins T, Shine R. (2010a) The physiological basis of geographic variation in rates of embryonic development within a widespread lizard species. Am Nat 176: 522–528. [DOI] [PubMed] [Google Scholar]
- 30.Du W-G, Ye H, Zhao B, Warner DA, Shine R. (2010b) Thermal acclimation of heart rates in reptilian embryos. PLoS One 5: e15308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du W, Zhao B, Shine R. (2010c) Embryos in the fast lane: hightemperature heart rates of turtles decline after hatching. PLoS One 5: e9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du W-G, Zhao B, Chen Y, Shine R. (2011) Behavioral thermoregulation by turtle embryos. Proc Natl Acad Sci USA 108: 9513–9515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dzialowski EM, O'Connor MP. (2001) Physiological control of warming and cooling during simulated shuttling and basking in lizards. Physiol Biochem Zool 74: 679–693. [DOI] [PubMed] [Google Scholar]
- 34.Ehrenberger JC. (2010) Physiological responses to temperature in the lizard, Sceloporus undulatus. MS Thesis Indiana State University, Terre Haute. [Google Scholar]
- 35.Francis C, Brooks GR. (1970) Oxygen consumption, rate of heart beat and ventilatory rate in parietalectomized lizards, Sceloporus occidentalis. Comp Biochem Physiol 35: 463–469. [Google Scholar]
- 36.Gilchrist GW, Huey RB. (1999) The direct response of Drosophila melanogaster to selection on knockdown temperature. Heredity 83: 15–29. [DOI] [PubMed] [Google Scholar]
- 37.Hansen J, Sato M, Ruedy R. (2012) Perception of climate change. Proc Natl Acad Sci USA 109: E2415–E2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann AA, Sørensen JG, Loeschcke V. (2003) Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J Therm Biol 28: 175–216. [Google Scholar]
- 39.Huey RB, Stevenson RD. (1979) Integrating thermal physiology and ecology of ectotherms: discussion of approaches. Am Zool 19: 357–366. [Google Scholar]
- 40.Huey RB, Crill WD, Kingsolver JG, Weber KE. (1992) A method for rapid measurement of heat or cold resistance of small insects. Funct Ecol 6: 489–494. [Google Scholar]
- 41.Huey RB, Deutsch CA, Tewksbury JJ, Vitt LJ, Hertz PE, Pérez HJA, Garland T. (2009) Why tropical forest lizards are vulnerable to climate warming. Proc Biol Sci 276: 1939–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.IPCC (2007) Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, Pachauri RK, Reisinger A, eds]. IPCC, Geneva, Switzerland, 104 pp. [Google Scholar]
- 43.Kearney M. (2011) Metabolic theory, life history and the distribution of a terrestrial ectotherm. Funct Ecol 26: 167–179. [Google Scholar]
- 44.Leaché AD, Reeder TW. (2002) Molecular systematics of the Eastern Fence Lizard (Sceloporus undulatus): a comparison of parsimony, likelihood, and Bayesian approaches. Syst Biol 51: 44–68. [DOI] [PubMed] [Google Scholar]
- 45.Leaché AD. (2009) Species tree discordance traces to phylogeographic clade boundaries in North American fence lizards (Sceloporus). Syst Biol 58: 547–559. [DOI] [PubMed] [Google Scholar]
- 46.Lee JY, Lee SJ. (2010) Thermal effect on heart rate and hemodynamics in vitelline arteries of stage 18 chicken embryos. J Biomech 43: 3217–3221. [DOI] [PubMed] [Google Scholar]
- 47.Licht P. (1965) Effects of temperature on heart rates of lizards during rest and activity. Physiol Zool 38: 129–137. [Google Scholar]
- 48.Licht P, Dawson WR, Shoemaker VH. (1966) Heat resistance of some Australian lizards. Copeia 1966: 162–169. [Google Scholar]
- 49.Lierz M, Gooss O, Hafez HM. (2006) Noninvasive heart rate measurement using a digital egg monitor in chicken and turkey embryos. J Avian Med Surg 20: 141–146. [Google Scholar]
- 50.Lutterschmidt WI, Hutchison VH. (1997) The critical thermal maximum: history and critique. Can J Zool 75: 1561–1574. [Google Scholar]
- 51.McGaugh SE, Schwanz LE, Bowden RM, Gonzalez JE, Janzen FJ. (2010) Inheritance of nesting behaviour across natural environmental variation in a turtle with temperature-dependent sex determination. Proc Biol Sci 277: 1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molnar PK, Kutz SJ, Hoar BM, Dobson AP. (2013) Metabolic approaches to understanding climate change impacts on seasonal host-macroparasite dynamics. Ecol Lett 16: 9–21. [DOI] [PubMed] [Google Scholar]
- 53.Mullins MA, Janzen FJ. (2006) Phenotypic effects of thermal means and variances on smooth softshell turtle (Apalone mutica) embryos and hatchlings. Herpetologica 62: 27–36. [Google Scholar]
- 54.Nechaeva MV. (2011) Physiological responses to acute changes in temperature and oxygenation in bird and reptile embryos. Respir Physiol Neurobiol 178: 108–117. [DOI] [PubMed] [Google Scholar]
- 55.Oufiero CE, Angilletta MJ. (2006) Convergent evolution of embryonic growth and development in the eastern fence lizard (Sceloporus undulatus). Evolution 60: 1066–1075. [PubMed] [Google Scholar]
- 56.Overall KL. (1994) Lizard egg environments. In Vitt LJ, Pianka ER, eds, Lizard Ecology. Princeton University Press, Princeton, pp 51–72. [Google Scholar]
- 57.Pinheiro J, Bates D, DebRoy S, Sarkar D, the R Development Core Team (2013) nlme: linear and nonlinear mixed effects models. R package version 3.1-109. [Google Scholar]
- 58.Qualls FJ, Shine R. (1998) Geographic variation in lizard phenotypes: importance of the incubation environment. Biol J Linn Soc 64: 477–491. [Google Scholar]
- 59.R Development Core Team (2011) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 60.Refsnider JM, Janzen FJ. (2012) Behavioural plasticity may compensate for climate change in a long-lived reptile with temperature-dependent sex determination. Biol Conserv 152: 90–95. [Google Scholar]
- 61.Schwarzkopf L, Andrews RM. (2012) “Selfish mothers” use “maternal manipulation” to maximize lifetime reproductive success. Herpetologica 68: 308–311. [Google Scholar]
- 62.Shine R, Elphick MJ, Harlow PS. (1997) The influence of natural incubation environments on the phenotypic traits of hatchling lizards. Ecology 78: 2559–2568. [Google Scholar]
- 63.Shine R, Downes SJ. (1999) Can pregnant lizards adjust their offspring phenotypes to environmental conditions? Oecologia 119: 1–8. [DOI] [PubMed] [Google Scholar]
- 64.Shine R, Elphick MJ, Barrott EG. (2003) Sunny side up: lethally high, not low, nest temperatures may prevent oviparous reptiles from reproducing at high elevations. Biol J Linn Soc 78: 325–334. [Google Scholar]
- 65.Shine R. (2004) Does viviparity evolve in cold climate reptiles because pregnant females maintain stable (not high) body temperatures? Evolution 58: 1809–1818. [DOI] [PubMed] [Google Scholar]
- 66.Shine R. (2012) Manipulative mothers and selective forces: the effects of reproduction on thermoregulation in reptiles. Herpetologica 68: 289–298. [Google Scholar]
- 67.Siddiqui WH, Barlow CA. (1972) Population growth of Drosophila melanogaster (Diptera: Drosophilidae) at constant and alternating temperatures. Ann Entomol Soc Am 65: 993–1001. [Google Scholar]
- 68.Siddiqui WH, Barlow CA. (1973) Population growth of Anagasta kuehniella (Lepidoptera: Pyralidae) at constant and alternating temperatures. Ann Entomol Soc Am 66: 579–585. [Google Scholar]
- 69.Siddiqui WH, Barlow CA, Randolph PA. (1973) Effects of some constant and alternating temperatures on population growth of the pea aphid, Acyrthosiphon pisum (Homoptera: Aphididae). Can Entomol 105: 145–156. [Google Scholar]
- 70.Stillman JH, Somero GN. (1996) Adaptation to temperature stress and aerial exposure in congeneric species of intertidal porcelain crabs (genus Petrolisthes): correlation of physiology, biochemistry and morphology with vertical distribution. J Exp Biol 199: 1845–1855. [DOI] [PubMed] [Google Scholar]
- 71.Storm MA, Angilletta MJ. (2007) Rapid assimilation of yolk enhances growth and development of lizard embryos from a cold environment. J Exp Biol 210: 3415–3421. [DOI] [PubMed] [Google Scholar]
- 72.Tazawa H. (2005) Cardiac rhythms in avian embryos and hatchlings. Avian Poult Biol Rev 16: 123–150. [Google Scholar]
- 73.Telemeco RS, Elphick MJ, Shine R. (2009) Nesting lizards (Bassiana duperreyi) compensate partly, but not completely, for climate change. Ecology 90: 17–22. [DOI] [PubMed] [Google Scholar]
- 74.Thompson MB, Packard GC, Packard MJ, Rose B. (1996) Analysis of the nest environment of tuatara Sphenodon punctatus. J Zool 238: 239–251. [Google Scholar]
- 75.Vleck CM, Vleck D. (1980) Patterns of metabolism and growth in avian embryos. Am Zool 20: 405–416. [Google Scholar]
- 76.Vleck CM, Hoyt DF. (1991) Metabolism and energetics of reptilian and avian embryos. In Deeming DC, Ferguson MWJ, eds, Egg Incubation: its Effects on Embryonic Development in Birds and Reptiles. Cambridge University Press, Cambridge, pp 285–306. [Google Scholar]
- 77.Warner DA, Andrews RM. (2002) Nest-site selection in relation to temperature and moisture by the lizard Sceloporus undulatus. Herpetologica 58: 399–407. [Google Scholar]
- 78.Warner DA, Jorgensen CF, Janzen FJ. (2010) Maternal and abiotic effects on egg mortality and hatchling size of turtles: temporal variation in selection over seven years. Funct Ecol 24: 857–866. [Google Scholar]
- 79.Warner DA, Shine R. (2011) Interactions among thermal parameters determine offspring sex under temperature-dependent sex determination. Proc Biol Sci 278: 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Winne CT, Keck MB. (2005) Intraspecific differences in thermal tolerance of the diamondback watersnake (Nerodia rhombifer): effects of ontogeny, latitude, and sex. Comp Biochem Physiol A 140: 141–149. [DOI] [PubMed] [Google Scholar]
- 81.Wood SN. (2004) Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Stat Assoc 99: 673–686. [Google Scholar]
- 82.Zuur AF, Leno EN, Walker N, Saveliev AA, Smith GM. (2009) Mixed Effects Models and Extensions in Ecology with R. Springer, New York. [Google Scholar]