Table 1.
Optimization of the zinc triflate catalyzed asymmetric ynamide addition to trifluoroacetophenone.
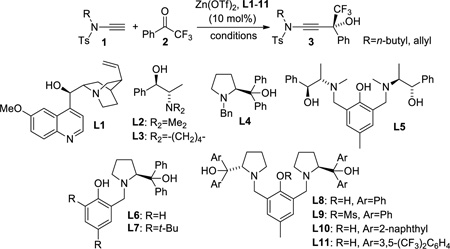 | |||||
|---|---|---|---|---|---|
| entry | ligand | R | conditions | yield (%) | ee (%) |
| 1 | L1 | n-Bu | DCE, 25 °C, 20 h | 75 | 48 |
| 2 | L2 | n-Bu | DCE, 25 °C, 20 h | 95 | 44 |
| 3 | L3 | n-Bu | DCE, 25 °C, 20 h | 89 | 33 |
| 4 | L4 | n-Bu | DCE, 25 °C, 16 h | 27 | 3 |
| 5 | L5 | n-Bu | DCE, 25 °C, 20 h | 15 | 22 |
| 6 | L6 | n-Bu | DCE, 25 °C, 16 h | 25 | 14 |
| 7 | L7 | n-Bu | DCE, 25 °C, 16 h | 72 | 45 |
| 8 | L8 | n-Bu | DCE, 25 °C, 16 h | 87 | 89 |
| 9 | L9 | n-Bu | DCE, 25 °C, 20 h | 15 | 0 |
| 10 | L10 | n-Bu | DCE, 25 °C, 16 h | 93 | 80 |
| 11 | L11 | n-Bu | DCE, 25 °C, 16 h | 22 | 9 |
| 12 | L8 | allyl | DCE, 25 °C, 16 h | 88 | 86 |
| 13 | L8 | n-Bu | CH2Cl2, 25 °C, 20 h | 95 | 87 |
| 14 | L8 | n-Bu | CHCl3, 25 °C, 20 h | 95 | 86 |
| 15 | L8 | n-Bu | toluene, 25 °C, 16 h | 70 | 78 |
| 16 | L8 | n-Bu | THF, 25 °C, 16 h | 55 | 66 |
| 17 | L8 | n-Bu | ACN, 25 °C, 20 h | 90 | 31 |
| 18 | L8 | n-Bu | EtOH, 25 °C, 20 h | 5 | n.d. |
| 19 | L8 | n-Bu | DCE, 0 °C, 45 h | 78 | 93 |
| 20 | L8 | n-Bu | DCE, −10 °C, 51 h | 73 | 91 |
| 21a | L8 | n-Bu | DCE, −20 °C, 24 h | 96 | 96 |
DCE=1,2-dichloroethane.
(EtO)3PO (20 mol%).
