Abstract
Respiratory tract injuries caused by inhalation of smoke or chemical products are related to significant morbidity and mortality. While many strategies have been built up to manage cutaneous burn injuries, few logical diagnostic strategies for patients with inhalation injuries exist and almost all treatment is supportive. The goals of initial management are to ensure that the airway allows adequate oxygenation and ventilation and to avoid ventilator-induced lung injury and substances that may complicate subsequent care. Intubation should be considered if any of the following signs exist: respiratory distress, stridor, hypoventilation, use of accessory respiratory muscles, blistering or edema of the oropharynx, or deep burns to the face or neck. Any patients suspected to have inhalation injuries should receive a high concentration of supplemental oxygen to quickly reverse hypoxia and to displace carbon monoxide from protein binding sites. Management of carbon monoxide and cyanide exposure in smoke inhalation patients remains controversial. Absolute indications for hyperbaric oxygen therapy do not exist because there is a low correlation between carboxyhemoglobin levels and the severity of the clinical state. A cyanide antidote should be administered when cyanide poisoning is clinically suspected. Although an ideal approach for respiratory support of patients with inhalation injuries do not exist, it is important that they are supported using techniques that do not further exacerbate respiratory failure. A well-organized strategy for patients with inhalation injury is critical to reduce morbidity and mortality.
Keywords: inhalation injury, burn, carbon monoxide poisoning, cyanide poisoning
Introduction
Respiratory tract injuries resulting from inhalation of smoke or chemical products are the leading causes of death in thermally injured patients.1 Inhalation injuries can lead to long-term pulmonary dysfunction. Smoke inhalation increases the incidence of respiratory complications such as pneumonia or acute respiratory distress syndrome.2
Shirani et al reported that the severity of an inhalation injury is directly proportionate to the area of the burn.2 Mortality of patients with an inhalation injury alone was expected to increase by a maximum of 20%, and by 60% with both inhalation injury and pneumonia. A meta-analysis on prognostic factors in patients with both cutaneous and inhalation injuries indicated that mortality increased significantly with inhalation injuries.3 While many strategies have been developed to manage cutaneous burn injuries, few logical diagnostic strategies for patients with inhalation injuries exist and almost all treatment is supportive.
Woodson indicated that the treatments for inhalation injuries have been improved less than the treatments for cutaneous burns.4 Some factors explain the delayed progress in the management of inhalation injuries. Burnt cutaneous tissue may be removed and replaced with skin grafts, but treatment of injured pulmonary tissue involves measures to prevent secondary injuries such as ventilator-induced lung injury to allow host mechanisms to repair injured tissue.
A potential limitation of inhalation injuries treatment is that uniform diagnostic criteria and severity scales have not been established.5 Current management methods include intubation in the emergency department, fiberoptic bronchoscopy, arterial blood gases, and mechanical ventilator support.1 This review presents the pathophysiology, diagnostic and management strategies in the emergency room.
Pathophysiology
Inhalation injuries are classified into three types: 1) upper airway injuries caused primarily by thermal injury to the mouth, oropharynx, and larynx; 2) lower airway and parenchymal injuries caused by chemical and particulate constituents of smoke; 3) metabolic asphyxiation, which is the process by which certain smoke constituents, such as carbon monoxide (CO) and cyanide, impair oxygen (O2) delivery to the tissue.6 It should be noted that these often overlap and that even patients without inhalation injury may develop massive facial edema requiring intubation during the resuscitation process.
Upper airway
Super-heated air usually injures only the airway structure above the vocal cords because of low heat capacity of air, efficient heat dissipation in the upper respiratory tract, and reflex closure of the upper airway. Injuries to the upper airway may induce massive edema of the tongue and epiglottis and obstruct the supraglottis.7 Airway edema occurs during the late phases of resuscitation.8 The initial evaluation is a poor indicator of the severity of airway swelling.
The need for airway control must always be assessed and intubation should be considered if any of the following significant injuries to the upper airway is suspected: respiratory distress, stridor, hypoventilation, use of accessory respiratory muscles, blistering or edema of the oropharynx, or deep burns to the face or neck.
Lower airway
The lower airway is damaged by smoke-related toxins, which are generated from the incomplete combustion of certain products.5 Burning cotton, rubber, and plastic produce many injurious substances such as the aldehydes, nitrogen dioxide, sulfur dioxide, ammonia, and chlorine, which turn into strong acids or alkalis when combined with water in the lower airway. These toxins damage epithelial and capillary cells of the tracheobronchial regions. This may result in increased alveolocapillary permeability, impaired mucociliary transport, ventilation perfusion mismatching and an increased susceptibility to respiratory infection.9–11
Mucociliary transportation is destroyed and the clearance of bacteria is reduced due to loss of the mucociliary elevator. The casts are formed with a combination of sloughed tissue, proteinaceous material leaking from the injured submucosa, and fibrin. These changes cause obstruction of the airway.12 These lesions progressively form primarily from fibrin and may be lethal by completely obstructing the respiratory tract.13
The second important factor is decreased lung compliance.14 This fall in compliance is associated with an increase in extravascular water. A retrospective study of 40 patients with smoke inhalation and/or cutaneous burn indicated that extravascular lung water increased significantly for more than 48 hours after injury in patients with smoke injury only. These reductions in compliance can greatly increase the work of breathing.15
The last factor is ventilation perfusion mismatching resulting from the immediate inactivation of surfactant, subsequent microatelectasis, and a consequence of small to medium caliber bronchiolar obstruction due to fibrin cast formation. A physiological shunt causes profound hypoxemia and acute microvascular injury in severe cases. These changes can produce the clinical features of acute respiratory distress syndrome.16
CO and cyanide
CO is a colorless, tasteless, inodorous, non-irritative gas produced by incomplete hydrogen combustion.17,18 CO poisoning is a major cause of morbidity in burn patients. It binds to hemoglobin with approximately 240 times greater affinity than O2.18 CO dissociates from hemoglobin less efficiently at the tissue level as a result of competing with O2 for hemoglobin binding, thus shifting the oxyhemoglobin dissociation curve to the left. O2 delivery to tissue is impaired because of reduced O2 carrying capacity and less efficiency dissociation at the tissue level. O2 utilization is also compromised because of impaired oxidative phosphorylation at the mitochondrial level.19 CO can induce myocardial injury because of direct injury to myoglobin. Furthermore, CO can precipitate lipid peroxidation by toxic O2 species that results in delayed neurologic sequelae.20
Hydrogen cyanide is produced during combustion of household materials containing both carbon and nitrogen. These include synthetic polymers, acrylonitrile, nylon, melamine, wool, and cotton. It inhibits oxidative phosphorylation, thereby stopping aerobic respiration and leads to anaerobic respiration and subsequent metabolic acidosis and cell death. These change are particularly deleterious to the cardiovascular and central nervous systems.21
Cyanide metabolism and neutralization involve a number of mechanisms. The most important is the conversion of cyanide to thiocyanide in the liver by rhodanese. Thiocyanide is excreted in the urine. A minor pathway for cyanide detoxification involves hydroxocobalamin, the precursor to vitamin B12. Circulating hydroxocobalamin combines with cyanide to form cyanocobalamin, which is safely excreted in the urine.
Cyanide poisoning is almost impossible to confirm without clinical suspicion. A low threshold should be maintained to empirically treat cyanide toxicity. The severity of cyanide poisoning depends on the amount of exposure, duration, and route. It mainly causes central nervous system and cardiovascular system dysfunction. Treatment for cyanide poisoning should be initiated in any patients with inhalation injury, unexplainable lactic acidosis, low arteriovenous O2 content difference, or high mixed venous O2. Cyanide poisoning should also be suspected if impaired consciousness cannot be explained only by CO poisoning. The frequency of cyanide-related inhalation injury has led to the use of a cyanide antidote kit.
Diagnosis
There is no consensus on the diagnostic criteria for inhalation injury. One of the reasons for the lack of consensus is that impaired pulmonary function due to inhalation injury often results from an inflammatory response to initial injury, and manifestations may be delayed for a day or two. It is also possible for thermally injured patients to experience acute lung injuries from systemic effects of the inflammation caused by severe cutaneous burns.22 Even if there is no evidence of respiratory distress, it is important to recognize features from past cases and physical examinations that reveal risk factors for inhalation injury. An early diagnosis is important in order to recognize a potentially compromised airway and to manage fluid resuscitation.
Inhalation injury is suspected clinically by direct observations and is often confirmed by additional diagnostic procedures such as bronchoscopy (Figure 1). These observations include a history of injury in a closed space fire, facial burns, and singeing nasal vibrissae.4 Physical findings including soot in the upper airways, hoarseness, and carbonaceous sputum may help support the diagnosis. Past cases include exposure to flames, blast injury, steam, or inhaled irritants. Furthermore, the effect of these items can be exacerbated by the duration of exposure. Physical examinations may identify visible injuries to the airway or evidence of pulmonary dysfunction. Diagnostic criteria for inhalation injuries are complicated by various manifestations between inhaled irritants and heated gas exposures, and distinguishing between them.23 The degree of smoke exposure may not be directly related to the severity of respiratory failure. Symptoms indicating bronchorrhea or bronchospasm, such as wheezing, cough, dyspnea, and prolonged expiratory time suggest direct toxin damage to the bronchial mucosa. When patients are considered at risk for upper airway thermal injury and occlusion, a priority is to evaluate the upper airway for impending occlusion that may be prevented by early tracheal intubation. Hypoxia, rales and rhonchi occur only in those with the most severe injuries.1 An admission chest X-ray is also a poor predictor, but is important for the baseline of evaluations.6,24
Figure 1.
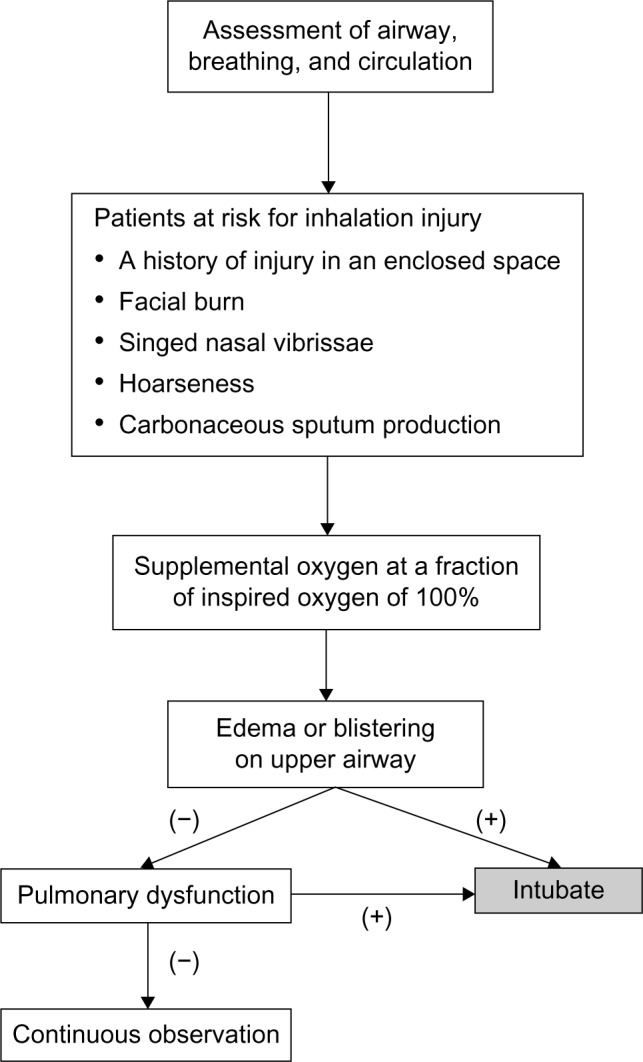
An algorithm to manage the patients at risk for inhalation injury in the emergency room.
The other tool presently available for diagnosis of inhalation injury is flexible fiberoptic bronchoscopy (Figure 1). Fiberoptic bronchoscopy allows direct visualization of tissue damage to the upper airway and identification of patients with compromised upper airways who need intubation.25 Bronchoscopic evidence of inhalation injuries includes soot deposits, erythema, edema, mucosal blisters and erosions, hemorrhages, and bronchorrhea.26,27 A scoring system for inhalation injury based on bronchoscopic evaluation has been used and it has been attempted to identify the relationship between these data and the prognoses. Endorf and Gamelli examined the effects of the degree of inhalation injury and partial pressure arterial oxygen/fraction of inspired oxygen (PaO2/FiO2 [P/F]) ratio on fluid requirements during acute resuscitation.26 Patients with more severe injuries identified during the initial bronchoscopy had a significantly worse chance of survival than those with mild injuries. These researchers or physicians also reported that high-grade injuries were not associated with increased fluid requirements. On the other hand, they concluded that the P/F ratio may be a more accurate predictor of increased fluid requirements during acute resuscitation.28,29 Chou et al examined the bronchoscopic classification of inhalation injury as a predictor of acute lung injury.27 Patients with deeper mucosal injuries on bronchoscopy had a significantly higher rate of acute lung injury. However, bronchoscopy performed soon after injury may not show mucosal injury in some cases.30 Diagnosis of inhalation injuries by bronchoscopy does not always identify patients who will experience progressive pulmonary dysfunction. Aggressive use of bronchoscopy is effective in airway hygiene, removing particulate matter, and accumulated secretion obstructing bronchi.6 A retrospective study showed that burn patients with inhalation injury and pneumonia who underwent bronchoscopy had decreased duration of mechanical ventilation, shorter length of stay in intensive care unit, and reduced mortality compared to patients who did not receive bronchoscopy.31
Radionuclide imaging such as 99-technetium scanning administered by inhalation can be used to diagnose inhalation injury. Asymmetric or delayed clearance may be indicated by the presence of small airway obstruction caused by airway debris, bronchospasm, or mucosal edema.32 These tests are highly sensitive and may confirm inhalation injury, but do not get used much as the initial evaluation of smoke inhalation due to logistic difficulty.33,34
Arterial blood gas should be measured for oxyhemoglobin saturation, carboxyhemoglobin concentration, and methemoglobin concentration to evaluate frequent concurrent injuries such as CO poisoning. CO poisoning is diagnosed with a suspicious history, physical examination, and an increased carboxyhemoglobin level. Severe metabolic acidosis with an elevated anion gap is expected in cyanide poisoning. Cyanide poisoning patients also have an elevated blood lactate concentration and narrowing of the venous-arterial gradient.35–37
A chest radiograph should be performed to assess parenchymal injury after inhalation, occult trauma, or aspiration. Although some authors reported that bronchial wall thickening was sometimes found on initial chest radiographs, patients with inhalation injuries often have a normal chest radiograph at initial stages.38 In contrast, the presence of pulmonary infiltrates at initial evaluation has been indicated as a marker of severe injury and a poor prognosis.39
Initial management
Immediate and directed assessment of the respiratory or circulatory status of patients with smoke inhalation is required similar to standard assessment and management for all trauma patients. Although few specific strategies for inhalation injury exist, appropriate initial management can influence a favorable outcome. The goals of initial management are to ensure that the airway allows adequate oxygenation and ventilation, and to avoid ventilator-induced lung injury and substances that may complicate subsequent care (Figure 1).
Intubation
Intubation should be considered if any of the following signs exist: respiratory distress, stridor, hypoventilation, use of accessory respiratory muscles, blistering or edema of the oropharynx, or deep burns to the face or neck (Figure 1). These findings show that the upper airway is at risk of progressive severe edema, which may compromise the patent airway. Patients with upper airway edema or hyperemia should be intubated promptly. Once intubated, the endotracheal tube should remain until the upper airway edema has disappeared.
In contrast, even if upper airway redness or edema is absent, patients should be kept under close observation for 24 hours especially the first 8 hours after injury. During the observation period, there should be a low threshold for intubation because upper airway obstruction with asphyxiation caused by progressive upper airway edema is a lethal complication of thermal injury.
O2
Any patients who have suspicious inhalation injuries should receive supplemental O2 at an FiO2 of 100% (Figure 1). Any comatose patients who have suspicious CO or cyanide poisoning should be ventilated mechanically using 100% O2. The purpose of such a high concentration of supplemental O2 is to quickly reverse hypoxia and to displace CO from hemoglobin. The next approach to presumed CO poisoning is to assess whether hyperbaric O2 therapy is indicated. The purpose of both supplemental oxygen and hyperbaric O2 therapy is to displace CO from hemoglobin.40
CO poisoning
CO exposure can cause cardiac injury even in those with normal coronary arteries (Figure 2). Cardiovascular investigation including electrocardiograms and measurement of cardiac enzymes may be required in exposed patients.41–43
Figure 2.
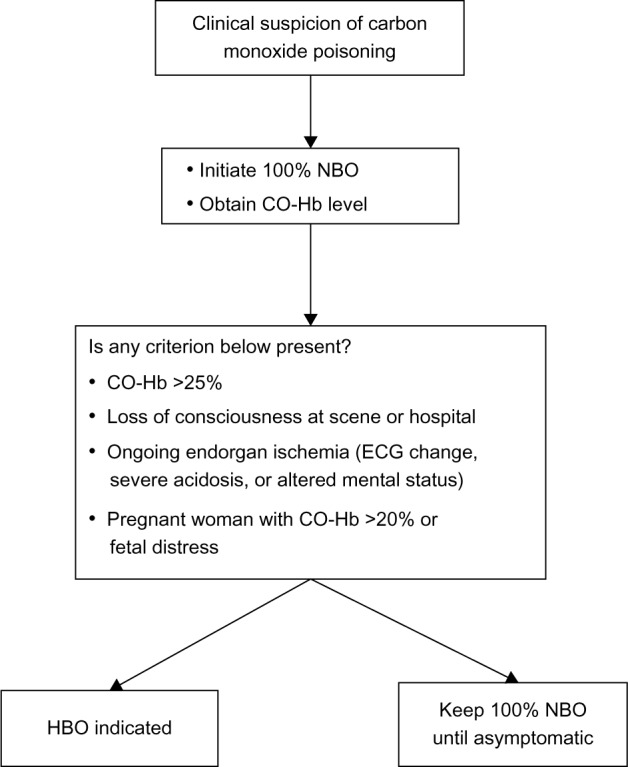
An algorithm to manage the patients at risk for carbon monoxide poisoning.
Abbreviations: CO-Hb, carboxyhemoglobin; ECG, electrocardiogram; NBO, normobaric oxygen; HBO, hyperbaric oxygen.
Absolute indications for hyperbaric O2 therapy remain controversial because there is a low correlation between carboxyhemoglobin levels and the severity of the outcomes.20 In addition, there is no gold standard of hyperbaric O2 treatment protocol. One single-center prospective trial showed that patients who underwent three hyperbaric O2 syndrome. Criteria for hyperbaric O2 therapy includes a carboxyhemoglobin level above 25%, loss of consciousness, evidence of ongoing end organ ischemia, or pregnant women with evidence of fetal distress or a carboxyhemoglobin level above 20%.44 A randomized trial showed that hyperbaric O2 treatment reduced the incidence of cognitive sequelae 6 weeks and 12 months after poisoning. Despite the work of these authors, larger multicenter work failed to demonstrate a benefit of hyperbaric O2 treatment.45,46 The carboxyhemoglobin level at which hyperbaric O2 therapy should be performed is controversial, regardless of clinical status. Recently, the Cochran group reviewed six randomized controlled trials of hyperbaric O2 therapy for the reduction of neurologic sequelae. Two studies showed a beneficial effect, while four studies did not. The investigators concluded that the efficacy of hyperbaric O2 therapy for reduction of the incidence of adverse neurologic outcomes is uncertain.47
Cyanide poisoning
Cyanide antidote should be administered when cyanide poisoning is clinically suspected. Cyanide antidote kits utilize oxidation of hemoglobin to methemoglobin and combination with cyanide to form cyanmethemoglobin. As cyanmethemoglobin disassociates, free cyanide is converted to thiocyanate using thiosulfate. And then, thiocyanate is excreted in the urine. Treatment with cyanide antidote kits is contraindicated in cases of concurrent CO toxicity.
Hydroxocobalamin is a precursor of vitamin B12, which forms cyanocobalamin after binding cyanide. Cyanocobalamin is stable and promptly excreted in the urine. Hydroxocobalamin is recommended as the first-line agent in cyanide poisoning.48 The dose of hydroxocobalamin administered intravenously is 70 mg/kg. If hydroxocobalamin is not available, another antidotal strategy involves the induction of methemoglobin. A relatively less toxic cyanmethemoglobin is formed, after cyanide binds methemoglobin. The induction of methemoglobinemia is accomplished by the administration of amyl nitrate and sodium nitrate. Sodium nitrate at 10 mg/kg and 1.65 mL/kg of 25% sodium thiosulfate should be administered intravenously. The use of the antidote kit is contraindicated in both inhalation injury and CO poisoning, whether together or separately, because the conversion of carboxyhemoglobin to methemoglobin may exacerbate hypoxia. It is only indicated in cases of isolated cyanide poisoning. Another strategy utilizes sodium thiosulfate for the conversion of cyanide.49
Mechanical ventilation
Patients who require intubation because of upper airway edema, pulmonary dysfunction, or impaired mental status generally require mechanical ventilation. Although an ideal approach for respiratory support of patients with inhalation injuries does not exist, there are many ways to administer positive pressure ventilation and many papers have been written advocating one method over another in those patients.
Positive end expiratory pressure (PEEP) is applied to improve hypoxemia related to alveolar hypoventilation. Improvement of hypoxemia as a result of intrapulmonary shunt requires interventions that open lung units for gas exchange. Optimal PEEP is the level of end expiratory pressure that may increase arterial oxygenation by preventing loss of lung compliance during mechanical ventilation, increasing functional residual capacity, and reducing venous admixture. PEEP levels should start at 8 cm H2O and be increased in 2.5 cm increments.
It is important that patients with inhalation injuries are supported using techniques that do not further exacerbate respiratory failure. Limitations of pressure and acceptance of permissive hypercapnia are important. To recruit injured lung, a pressure-based strategy may be far more effective. The tidal volumes should be initiated at 6–8 mL/kg of predicted body weight. The plateau airway pressure of less than 30 cm H2O should be considered.50 Ventilator protocol relating to hypercapnia is acceptable within clinically practical hemodynamic bounds. Permissive hypercapnia can be used if the targeted pH is above 7.25. At greater degrees of hypercapnia and acidosis, hemodynamic instability may be a limiting factor.51
There are some inhaled adjunctive agents, such as inhaled heparin, N-acetylcysteine, and beta-agonist. N-acetylcysteine is a mucolytic agent, which ruptures the mucoprotein in mucus. A retrospective study showed that heparin in combination with the use of N-acetylcysteine decreased re-intubation rates and improved mortality in pediatric patients with inhalation injury.52 However, another retrospective study indicated that treatment with N-acetylcysteine and heparin did not improve outcomes of patients with inhalation injury.53 It is still unclear whether nebulized heparin or N-acetylcysteine improves outcomes of patients with inhalation injury. Inhaled bronchodilators such as beta-agonist may help manage bronchoconstriction caused in the injured lower airways by inhalation injury. In ovine models of inhalation injury, the use of nebulized albuterol or epinephrine caused a decrease in airway pressure and an improvement in P/F ratio.54,55
Escharotomy may be required for patients with circumferential full-thickness burns of the anterolateral torso. In these patients, edema builds up under eschar during the resuscitation period, gradually constricting chest excursion and causing increased peak expiratory pressure.56 The treatment is thoracic escharotomy in either emergency room or operating theater, which results in restoration of chest compliance.
Footnotes
Disclosure
The author has no conflicts of interest to disclose.
References
- 1.Mlcak RP, Suman OE, Herndon DN. Respiratory management of inhalation injury. Burns. 2007;33(1):2–13. doi: 10.1016/j.burns.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Shirani KZ, Pruitt BA, Jr, Mason AD., Jr The influence of inhalation injury and pneumonia on burn mortality. Ann Surg. 1987;205(1):82–87. doi: 10.1097/00000658-198701000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colohan SM. Predicting prognosis in thermal burns with associated inhalational injury: a systematic review of prognostic factors in adult burn victims. J Burn Care Res. 2010;31(4):529–539. doi: 10.1097/BCR.0b013e3181e4d680. [DOI] [PubMed] [Google Scholar]
- 4.Woodson LC. Diagnosis and grading of inhalation injury. J Burn Care Res. 2009;30(1):143–145. doi: 10.1097/BCR.0b013e3181923b71. [DOI] [PubMed] [Google Scholar]
- 5.Palmieri TL. Inhalation injury: research progress and needs. J Burn Care Res. 2007;28(4):549–554. doi: 10.1097/BCR.0B013E318093DEF0. [DOI] [PubMed] [Google Scholar]
- 6.Cancio LC. Airway management and smoke inhalation injury in burn patient. Clin Plast Surg. 2009;36(4):555–567. doi: 10.1016/j.cps.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Moritiz AR, Henriques FC, McLean R. The effects of inhaled heat on the air passages and lungs: an experimental investigation. Am J Pathol. 1945;21(2):311–331. [PMC free article] [PubMed] [Google Scholar]
- 8.Haponik EF, Munster AM, Wise RA, et al. Upper airway function in burn patients. Correlation of flow-volume curves and nasopharyngoscopy. Am Rev Respir Dis. 1984;129(2):251–257. [PubMed] [Google Scholar]
- 9.Pruitt BA, Jr, Cioffi WG, Shimazu T, Ikeuchi H, Mason AD., Jr Evaluation and management of patients with inhalation injury. J Trauma. 1990;30(12 Suppl):S63–S68. doi: 10.1097/00005373-199012001-00015. [DOI] [PubMed] [Google Scholar]
- 10.Herndon DN, Traber DL, Niehaus GD, Linares HA, Traber LD. The pathophysiology of smoke inhalation injury in a sheep model. J Trauma. 1984;24(12):1044–1051. doi: 10.1097/00005373-198412000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Stollery DE, Jones RL, King EG. Deadspace ventilation: a significant factor in respiratory failure after thermal inhalation. Crit Care Med. 1987;15(3):260–261. [PubMed] [Google Scholar]
- 12.Walker HL, McLeod CG, Jr, McManus WF. Experimental inhalation injury in the goat. J Trauma. 1981;21(11):962–964. doi: 10.1097/00005373-198111000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Venus B, Matsuda T, Copiozo JB, Mathru M. Prophylactic intubation and continuous positive airway pressure in the management of inhalation injury in burn victims. Crit Care Med. 1981;9(7):519–523. doi: 10.1097/00003246-198107000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Neiman GF, Clark WR, Jr, Wax SD, Webb SR. The effect of smoke inhalation on pulmonary surfactant. Ann Surg. 1981;191(2):171–181. doi: 10.1097/00000658-198002000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herndon DN, Barrow RE, Traber DL, Rutan TC, Rutan RL, Abston S. Extravascular lung water changes following smoke inhalation and massive burn injury. Surgery. 1987;102(2):341–349. [PubMed] [Google Scholar]
- 16.Robinson NB, Hudson LD, Robertson HT, Thorning DR, Carrico CJ, Heimbach DM. Ventilation and perfusion alterations after smoke inhalation injury. Surgery. 1981;90(2):352–363. [PubMed] [Google Scholar]
- 17.Weaver LK. Carbon monoxide poisoning. Crit Care Clin. 1999;15(2):297–317. doi: 10.1016/s0749-0704(05)70056-7. [DOI] [PubMed] [Google Scholar]
- 18.McCall JE, Cahill TJ. Respiratory care of the burn patient. J Burn Care Rehabil. 2005;26(3):200–206. [PubMed] [Google Scholar]
- 19.Hardy KR, Thom SR. Pathophysiology and treatment of carbon monoxide poisoning. J Toxicol Clin Toxicol. 1994;32(6):613–629. doi: 10.3109/15563659409017973. [DOI] [PubMed] [Google Scholar]
- 20.Kealey GP. Carbon monoxide toxicity. J Burn Care Res. 2009;30(1):146–147. doi: 10.1097/BCR.0b013e3181923b81. [DOI] [PubMed] [Google Scholar]
- 21.Lawson-Smith P, Jansen EC, Hyldegaard O. Cyanide intoxication as part of smoke inhalation-a review on diagnosis and treatment from the emergency perspective. Scand J Trauma Resusc Emerg Med. 2011;19:14. doi: 10.1186/1757-7241-19-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinvall I, Bak Z, Sjoberg F. Acute respiratory distress syndrome is as important as inhalation injury for the development of respiratory dysfunction in major burns. Burns. 2008;34(4):441–451. doi: 10.1016/j.burns.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Palmieri TL, Klein MB. Burn research state of the science: introduction. J Burn Care Res. 2007;28(4):544–545. doi: 10.1097/BCR.0b013e318095a40f. [DOI] [PubMed] [Google Scholar]
- 24.Putman CE, Loke J, Matthay RA, Ravin CE. Radiographic manifestations of acute smoke inhalation. AJR Am J Roentgenol. 1977;129(5):865–870. doi: 10.2214/ajr.129.5.865. [DOI] [PubMed] [Google Scholar]
- 25.Muehlberger T, Kunar D, Munster A, Couch A. Efficacy of fiberoptic laryngoscopy in the diagnosis of inhalation injuries. Arch Otolaryngol Head Neck Surg. 1998;124(9):1003–1007. doi: 10.1001/archotol.124.9.1003. [DOI] [PubMed] [Google Scholar]
- 26.Endorf FW, Gamelli RL. Inhalation injury, pulmonary perturbations, and fluid resuscitation. J Burn Care Res. 2007;28(1):80–83. doi: 10.1097/BCR.0B013E31802C889F. [DOI] [PubMed] [Google Scholar]
- 27.Chou SH, Lin SD, Chuang HY, Cheng YJ, Kao EL, Huang MF. Fiber-optic bronchoscopic classification of inhalation injury: prediction of acute lung injury. Surg Endosc. 2004;18(9):1377–1379. doi: 10.1007/s00464-003-9234-2. [DOI] [PubMed] [Google Scholar]
- 28.Mosier MJ, Pham TN, Park DR, Simmons J, Klein MB, Gibran NS. Predictive value of bronchoscopy in assessing the severity of inhalation injury. J Burn Care Res. 2012;33(1):65–73. doi: 10.1097/BCR.0b013e318234d92f. [DOI] [PubMed] [Google Scholar]
- 29.Albright JM, Davis CS, Bird MD, et al. The acute pulmonary inflammatory response to the graded severity of smoke inhalation injury. Crit Care Med. 2012;40(4):1113–1121. doi: 10.1097/CCM.0b013e3182374a67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunt JL, Agee RN, Pruitt BA., Jr Fiberoptic bronchoscopy in acute inhalation injury. J Trauma. 1975;15(8):641–649. doi: 10.1097/00005373-197508000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Carr JA, Phillips BD, Bowling WM. The utility of bronchoscopy after inhalation injury complicated by pneumonia in burn patients: results from the National Burn Repository. J Burn Care Res. 2009;30(6):967–974. doi: 10.1097/BCR.0b013e3181bfb77b. [DOI] [PubMed] [Google Scholar]
- 32.Lull RJ, Tatum JL, Sugerman HJ, Hartshorne MF, Boll DA, Kaplan KA. Radionuclide evaluation of lung trauma. Semin Nucl Med. 1983;13(3):223–237. doi: 10.1016/s0001-2998(83)80017-8. [DOI] [PubMed] [Google Scholar]
- 33.Shiau YC, Liu FY, Tsai JJ, Wang JJ, Ho ST, Kao A. Usefulness of technetium-99m hexamethylpropylene amine oxime lung scan to detect inhalation lung injury of patients with pulmonary symptoms/signs but negative chest radiograph and pulmonary function test findings after a fire accident – a preliminary report. Ann Nucl Med. 2003;17(6):435–438. doi: 10.1007/BF03006430. [DOI] [PubMed] [Google Scholar]
- 34.Lin WY, Kao CH, Wang SJ. Detection of acute inhalation injury in fire victims by means of technetium-99m DTPA radioaerosol inhalation lung scintigraphy. Eur J Nucl Med. 1997;24(2):125–129. doi: 10.1007/BF02439543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baud FJ, Borron SW, Mégarbane B, et al. Value of lactic acidosis in the assessment of the severity of acute cyanide poisoning. Crit Care Med. 2002;30(9):2044–2055. doi: 10.1097/00003246-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Baud FJ, Barriot P, Toffis V, et al. Elevated blood cyanide concentration in victims of smoke inhalation. N Engl J Med. 1991;325(25):1761–1766. doi: 10.1056/NEJM199112193252502. [DOI] [PubMed] [Google Scholar]
- 37.Johnson RP, Mellors JW. Arteriolization of venous blood gases: a clue to the diagnosis of cyanide poisoning. J Emerg Med. 1988;6(5):401–405. doi: 10.1016/0736-4679(88)90014-5. [DOI] [PubMed] [Google Scholar]
- 38.Lee MJ, O’Connell DJ. The plain chest radiograph after acute smoke inhalation. Clin Radiol. 1988;39(1):33–37. doi: 10.1016/s0009-9260(88)80334-9. [DOI] [PubMed] [Google Scholar]
- 39.Masanès MJ, Legendre C, Lioret N, Saizy R, Lebeau B. Using bronchoscopy and biopsy of diagnose early inhalation injury. Macroscopic and histologic findings. Chest. 1995;107(5):1365–1369. doi: 10.1378/chest.107.5.1365. [DOI] [PubMed] [Google Scholar]
- 40.Weaver LK, Howe S, Hopkins R, Chan KJ. Carboxyhemoglobin half-life in carbon monoxide-poisoned patients treated with 100% oxygen at atmospheric pressure. Chest. 2000;117(3):801–808. doi: 10.1378/chest.117.3.801. [DOI] [PubMed] [Google Scholar]
- 41.Kalay N, Ozdogru I, Cetinkaya Y, et al. Cardiovascular effects of carbon monoxide poisoning. Am J Cardiol. 2007;99(3):322–324. doi: 10.1016/j.amjcard.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 42.Henry CR, Satran D, Lindgren B, Adkinson C, Nicholson CI, Henry TD. Myocardial injury and long-term mortality following moderate to severe carbon monoxide poisoning. JAMA. 2006;295(4):398–402. doi: 10.1001/jama.295.4.398. [DOI] [PubMed] [Google Scholar]
- 43.Satran D, Henry CR, Adkinson C, Nicholson CI, Bracha Y, Henry TD. Cardiovascular manifestations of moderate to severe carbon monoxide poisoning. J Am Coll Cardiol. 2005;45(9):1513–1516. doi: 10.1016/j.jacc.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 44.Kao LW, Nañagas KA. Carbon monoxide poisoning. Emerg Med Clin North Am. 2004;22(4):985–1018. doi: 10.1016/j.emc.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Weaver LK, Hopkins RO, Chan KJ, et al. Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J Med. 2002;347(14):1057–1067. doi: 10.1056/NEJMoa013121. [DOI] [PubMed] [Google Scholar]
- 46.Annane D, Chadda K, Gajdos P, Jars-Guincestre MC, Chevret S, Raphael JC. Hyperbaric oxygen therapy for acute domestic carbon monoxide poisoning: two randomized controlled trials. Intensive Care Med. 2011;37(3):486–492. doi: 10.1007/s00134-010-2093-0. [DOI] [PubMed] [Google Scholar]
- 47.Buckley NA, Juurlink DN, Isbister G, Bennett MH, Lavonas EJ. Hyperbaric oxygen for carbon monoxide poisoning. Cochrane Database Syst Rev. 2011;4:CD002041. doi: 10.1002/14651858.CD002041.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dumestre D, Nickerson D. Use of cyanide antidotes in burn patients with suspected inhalation injuries in North America: a cross-sectional survey. J Burn Care Res. 2014;35(2):e112–e117. doi: 10.1097/BCR.0b013e31829b3868. [DOI] [PubMed] [Google Scholar]
- 49.Barillo DJ. Diagnosis and treatment of cyanide toxicity. J Burn Care Res. 2009;30(1):148–152. doi: 10.1097/BCR.0b013e3181923b91. [DOI] [PubMed] [Google Scholar]
- 50.Latenser BA. Critical care of the burn patients: the first 48 hours. Crit Care Med. 2009;37(10):2819–2826. doi: 10.1097/CCM.0b013e3181b3a08f. [DOI] [PubMed] [Google Scholar]
- 51.Dries DJ. Key questions in ventilator management of the burn-injured patient (first of two parts) J Burn Care Res. 2009;30(1):128–138. doi: 10.1097/BCR.0b013e318191fe44. [DOI] [PubMed] [Google Scholar]
- 52.Desai MH, Mlcak R, Richardson J, Nichols R, Herndon DN. Reduction in mortality in pediatric patients with inhalation injury with aerosolized heparin/N-acetylcystine therapy. J Burn Care Rehabil. 1998;19(3):210–212. doi: 10.1097/00004630-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 53.Holt J, Saffle JR, Morris SE, Cochran A. Use of inhaled heparin/N-acetylcystine in inhalation injury: does it help? J Burn Care Res. 2008;29(1):192–195. doi: 10.1097/BCR.0b013e31815f596b. [DOI] [PubMed] [Google Scholar]
- 54.Lange M, Hamahata A, Traber DL, et al. Preclinical evaluation of epinephrine nebulization to reduce airway hyperemia and improve oxygenation after smoke inhalation injury. Crit Care Med. 2011;39(4):718–724. doi: 10.1097/CCM.0b013e318207ec52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palmieri TL, Enkhbaatar P, Bayliss R, et al. Continuous nebulized albuterol attenuates acute lung injury in an ovine model of combined burn and smoke inhalation. Crit Care Med. 2006;34(6):1719–1724. doi: 10.1097/01.CCM.0000217215.82821.C5. [DOI] [PubMed] [Google Scholar]
- 56.Greenhalgh DG, Warden GD. The importance of intra-abdominal pressure measurements in burned children. J Trauma. 1994;36(5):685–690. doi: 10.1097/00005373-199405000-00015. [DOI] [PubMed] [Google Scholar]