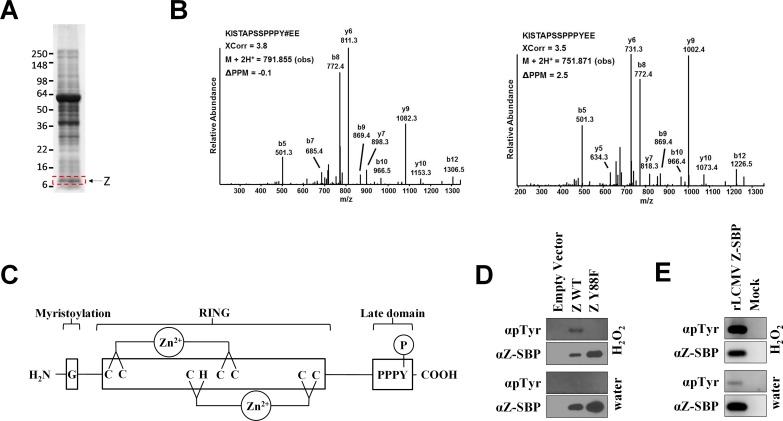
Fig 1. The LCMV matrix protein PPXY late domain is reversibly phosphorylated.
(A) Protein lysates from sucrose-banded LCMV strain Armstrong 53b particles were separated on polyacrylamide gels and stained with Coomassie brilliant blue. A gel slice containing the Z protein (indicated by the red box) was excised, subjected to in-gel tryptic and/or chymotryptic digestion, and extracted peptides were analyzed by mass spectrometry for the presence of phosphorylated peptides as described in the Materials and Methods. (B) Representative low energy collision-induced dissociation tandem mass spectra of a chymotryptic peptide harboring the indicated phosphotyrosine residue or the same peptide unphosphorylated. Both peptides were identified from virion-derived LCMV Z protein. The tandem mass spectra were collected in an Orbitrap (MS1)-linear ion trap (MS2) mass spectrometer. Y# denotes phosphotyrosine. The SEQUEST XCorr values, the precursor observed mass and the associated PPM are indicated. S1 Fig shows the corresponding calculated and measured b- and y-type ions indicating identified fragment ion masses. (C) Depiction of the LCMV Z protein. G, glycine at position 2 that is myristoylated; RING, the central zinc-binding really interesting new gene (RING) domain; PPPY, LCMV’s only known late domain that contains the Y88 site of phosphorylation. (D) Tyrosine 88 in the LCMV matrix protein is phosphorylated. HEK293T cells were transfected with an empty vector or a plasmid encoding LCMV Z (either WT or Y88F) with a C-terminal streptavidin binding peptide (SBP) tag. Following a 15 minute exposure to either water or the tyrosine phosphatase inhibitor, H2O2, Z was affinity purified from cell lysates using magnetic streptavidin beads and screened via western blot using antibodies specific for phosphotyrosine or the SBP tag. Results are representative of 3 independent experiments. (E) LCMV is phosphorylated in rodent cells. L929 cells were infected or not with a rLCMV that encodes a streptavidin binding peptide (SBP) fusion tag at the C terminus of Z. Two days later, cells were exposed to either water or the tyrosine phosphatase inhibitor, H2O2, for 15 minutes. SBP-tagged Z was then affinity purified from cell lysates using magnetic streptavidin beads and screened via western blot using antibodies specific for phosphotyrosine or the SBP tag. Results are representative of 3 independent experiments.