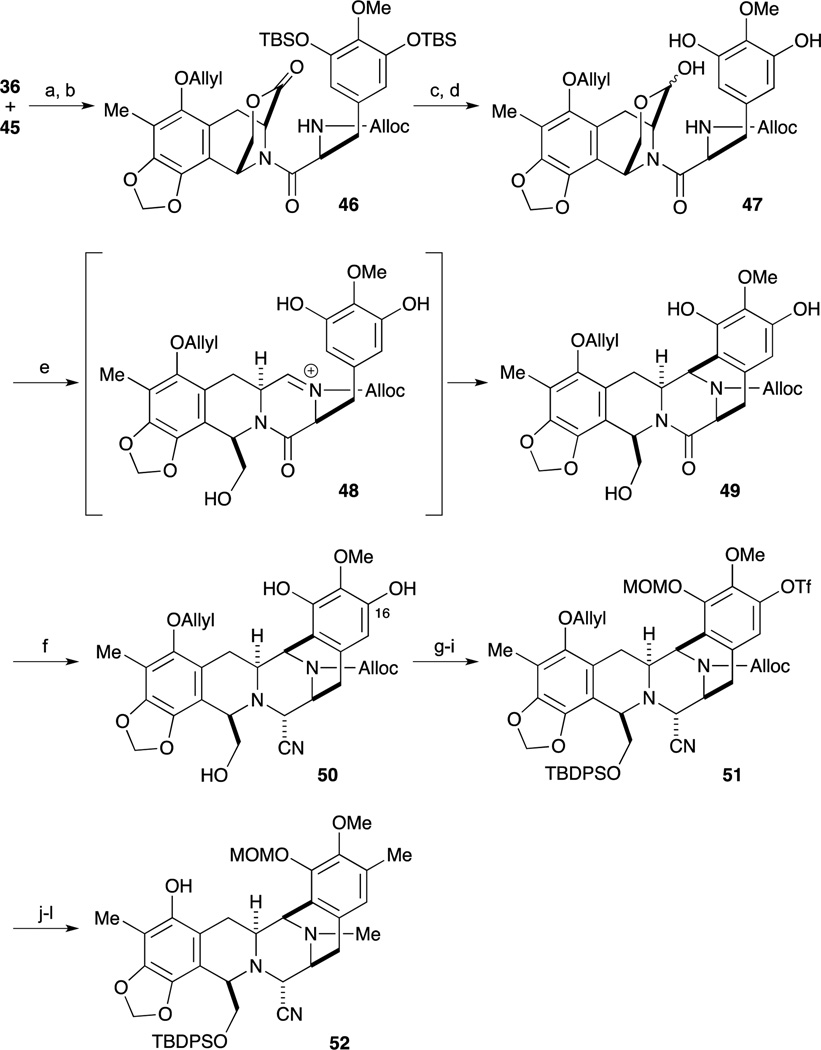
Scheme 4.
Reagents and conditions (a) 2-chloro-1,3-dimethyl-imidazolidium hexafluorophosphate (CIP), HOAt, Et3N, THF/CH2Cl2, 23 °C; (b) bromide, Cs2CO3, DMF, 23 °C (81%, 2 steps); (c) LiAlH2(OEt)2, Et2O, –78 °C (95%); (d) KF, MeOH, 23 °C; (e) 0.6 M TfOH, H2O/CF3CH2OH, BHT, 45 °C (89%, 2 steps); (f) LiAlH2(OEt)2, THF, 0 °C; AcOH, 4.8 M aq KCN, 23 °C (87%); (g) PhNTf2, Et3N, DMAP, CH2Cl2, –30 °C (74%); (h) TBDPSCl, DMAP, CH2Cl2, 23 °C (89%); (i) MOMBr, DIPEA, CH2Cl2, 23 °C (92%); (j) n-Bu3SnH, PdCl2(PPh3)2, AcOH, CH2Cl2, 23 °C (quant); (k) CH2O, NaBH3CN, AcOH, MeCN, 23 °C (95%); (l) SnMe4, PdCl2(PPh3)2, LiCl, DMF, 80 °C (83%).