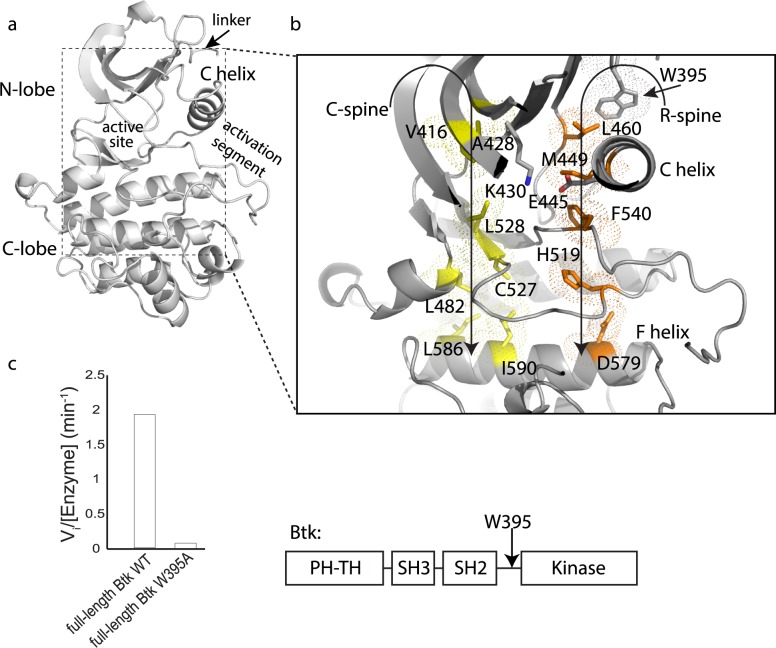
Fig 1. W395 is required for Btk activity.
(a) Btk linker-kinase domain structure (PDB ID: 3K54) showing the linker, N- and C-lobes, active site, and activation segment. (b) Key regulatory elements in the Btk linker-kinase domain are the R- and C-spines, orange and yellow, respectively. ATP completes the C-spine structure in the N-lobe but is omitted here for clarity. W395 is shown above the αC-helix and the residues in the conserved salt bridge, K430 and E445 are labeled. The C- and R-spines are supported by the αF-helix in the C-lobe of the kinase domain. (c) Initial velocity measurements comparing the activity of full-length Btk (domain structure is shown to the right) to full-length Btk (W395A) using the poly (4:1, Glu:Tyr) peptide substrate [11].