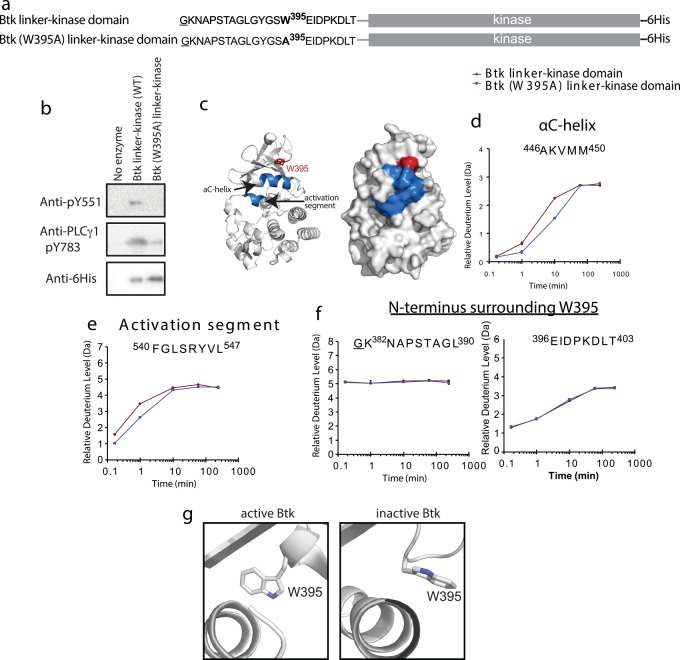
Fig 2. HDXMS reveals greater conformational sampling for active Btk linker-kinase domain.
(a) Constructs used for activity assays and HDXMS study. The underlined residue is vector derived and not part of the sequence of the Btk kinase domain. Both Btk linker-kinase and Btk (W395A) linker-kinase carried a hexahistidine tag (6His) at the C-terminus. (b) Western blot assay to probe phosphorylation of the Btk activation loop Y551 and Y783 in the PLCγ1 substrate. Anti-His antibody recognizes the 6-His tag on the Btk constructs and is used to detect the amount of Btk enzyme in each reaction. (c) Differences greater than 0.7 Da at any one of the five time points between 10 seconds and 4 hours in the hydrogen-deuterium exchange experiment are mapped onto two depictions of the Btk linker-kinase domain (PDB ID: 3GEN) and colored in blue. The side chain of W395 is red and labeled. (d-e) Deuterium exchange for peptides derived from the αC-helix and activation segment in Btk linker-kinase (red) and Btk (W395A) linker-kinase (blue). (f) Deuterium exchange for peptides derived from the linker and N-terminal region of Btk linker-kinase (red) and Btk (W395A) linker-kinase (blue). Complete HDX data is provided in Fig A in S1 Text. (g) Side-chain rotamer conformations of W395 in the structures of active (3K54) and inactive (3GEN) Btk linker-kinase.