Fig 2. Fractionation on a MonoQ anion exchange column of maltase activity from endosperm.
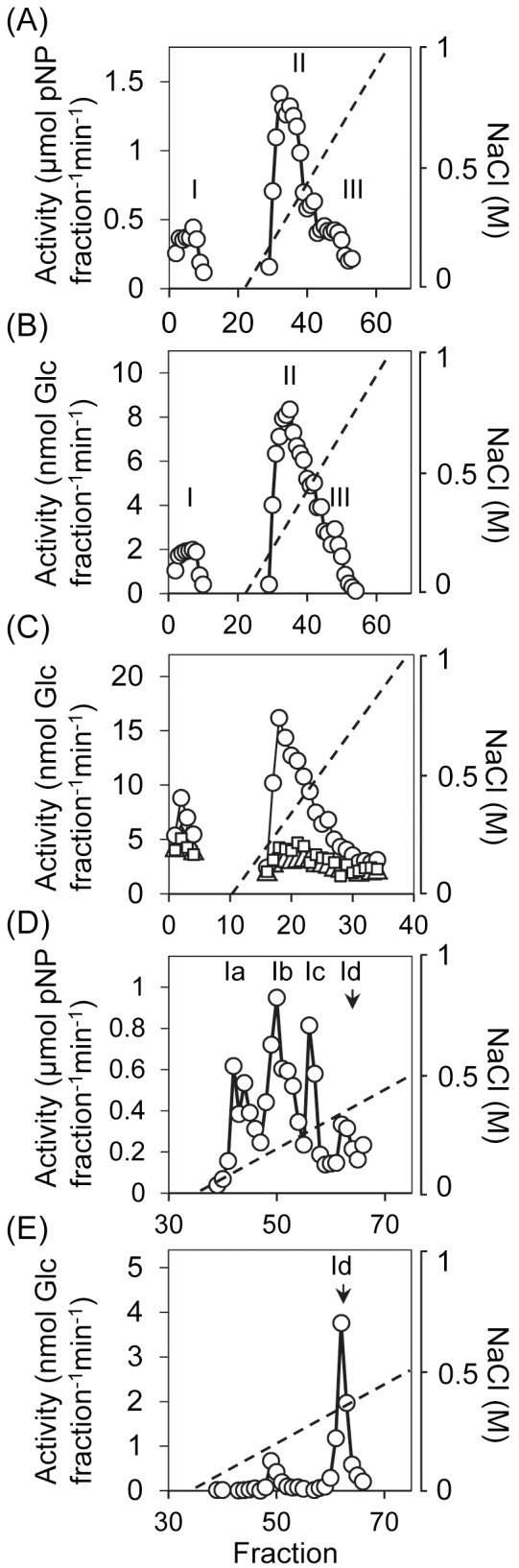
All fractionations were reproduced at least twice, on independent batches of seedlings. Representative examples are shown. (A), (B). Extracts of endosperm of seedlings of cv NFC Tipple at 10 dpi were applied to a MonoQ column by FPLC. Proteins binding the column were eluted with a gradient of increasing NaCl concentration (dashed line). One-mL fractions were collected and assayed for α-glucosidase activity with pNPG (A), or maltase activity with maltose (B). I-III indicate the three peaks of activity. (C). Extracts were prepared, fractionated and assayed for maltase as in (B), except that seedlings were either transgenic lines in a cv Golden Promise background carrying an RNA interference (RNAi) silencing cassette for Agl97 [lines 21 and 23 [31] (squares)], or an out-segregant line (i.e. not carrying the silencing cassette) from the same transformation experiment (circles). Chromatographic conditions were the same as in (B) except that the column wash to remove unbound proteins was reduced from 20 to 10 mL. Note that peak II maltase activity elutes at the same NaCl concentration in the experiments shown in (B) and (C). (D), (E). Fractionation on a MonoS column of proteins not binding to a MonoQ column [peak I in (A) and (B)]. Proteins binding to the MonoS column were eluted with a gradient of increasing NaCl concentration (dashed line). One-mL fractions were collected and assayed for α-glucosidase activity with pNPG (D), or for maltase activity with maltose (E). Peaks of activity eluting from the column are designated Ia-Id.
