The purpose of cardiovascular molecular imaging is to detect, characterize, and quantify relevant pathobiological processes at molecular and cellular levels in humans and other living systems.1 Thus, molecular imaging can be perceived best in contrast with anatomical imaging, where the focus is on the structure (as opposed to a process), and imaging of tissues or cells outside of the living systems. By this definition, cardiac CT to detect coronary calcification is not a molecular imaging technique, but 18F-NaF PET imaging of the calcification process in coronary arteries is a prototypical example of cardiovascular molecular imaging. These boundaries are somewhat blurry regarding physiological imaging. The quantification of left ventricular ejection fraction using various imaging modalities is clearly outside the realm of molecular imaging. However, whether or not to classify other examples of physiological imaging as molecular imaging can be debatable. As such, myocardial perfusion imaging is considered by many as an early example of molecular imaging in cardiovascular medicine. For the sake of clarity, we prefer to reserve the term “molecular imaging” for techniques that target cellular and molecular processes directly. With this narrower definition, 18F-fluorodeoxyglucose imaging of myocardial metabolism is molecular imaging, while myocardial perfusion imaging is not.
Fundamentally, cardiovascular molecular imaging is multidisciplinary. Related fields range from clinical cardiovascular medicine (identification of a diagnostic gap) to biology (identification of potential targets), chemistry (generation of ligands and development of molecular imaging probes), physics (instrumentation, probe contrast detection and signal processing) and data processing (Figure 1). A comprehensive insight into these components is required for meaningful analysis of molecular imaging data, at least in the early stages of development, and their validation prior to translation form preclinical studies to clinical trials and ultimately clinical practice.
Figure 1.
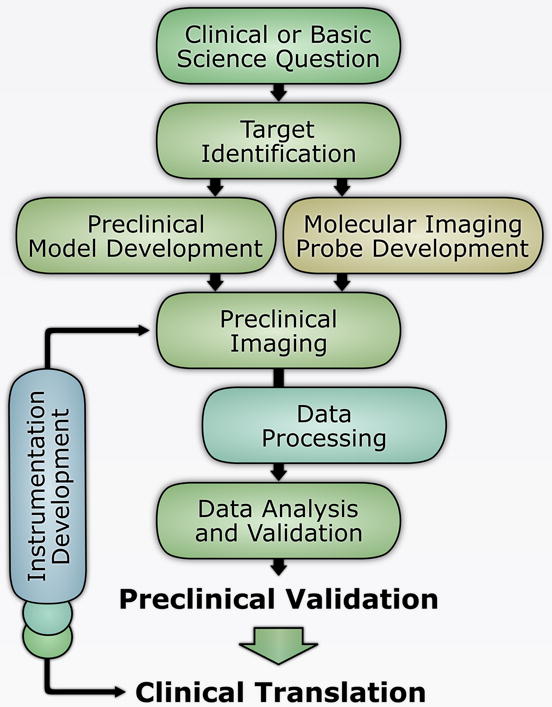
Multidisciplinary steps in the development of novel molecular imaging techniques.
The promise of molecular imaging is in personalized, precision, medicine and improving patient care by addressing diagnostic gaps that traditional tests have failed to address optimally. Evaluation (not necessarily imaging) of atherosclerotic plaque vulnerability, improved assessment of rupture risk in aortic aneurysm, sudden death risk stratification in heart failure, phenotypic characterization of heart failure with preserved ejection fraction, and identification of subjects with early stage calcific aortic valve disease who are prone to developing aortic stenosis are examples of such diagnostic gaps, just to name a few. By targeting specific biological properties of the tissue (e.g. tissue inflammation, via targeting of molecules specifically associated with the presence of inflammatory cells), and thus providing information on key pathophysiologic processes, clinical implementation of molecular imaging can lead to early detection of cardiovascular diseases (prior to structural changes detectable by other imaging modalities), evaluate their severity and potential for progression over time, and assess the effect of therapeutic interventions to guide patient management (Figure 2).
Figure 2.
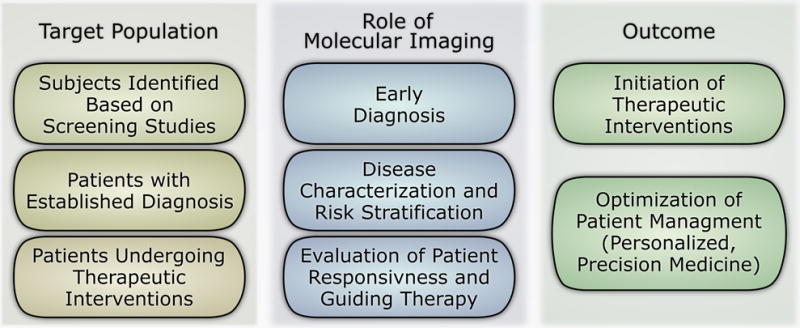
Roles of molecular imaging in patient management.
Schematically, molecular imaging often involves the administration of a targeted, labeled probe (tracer), detection of the probe with an appropriate imaging system, and generation of images that reflect the probe distribution, which is expected to mirror the biological properties of the imaged tissues (Figure 3). The nature of the probe can vary widely based on the imaging modalities used and the study design. The key is the probe’s ability to target a molecular process specifically and detectability by an imaging modality. The probe pharmacokinetics is of critical importance. Indeed, tissue biodistribution of the probe depends on plasma concentration (itself a function of metabolism rate and excretion), regional blood flow, probe diffusion, specific and non-specific interactions within the tissue, cellular internalization, and possibly intracellular interactions and modifications. The presence of a non-specific signal within or in the vicinity of the target tissue can adversely affect the visualization and/or quantification of the specific signal.
Figure 3.
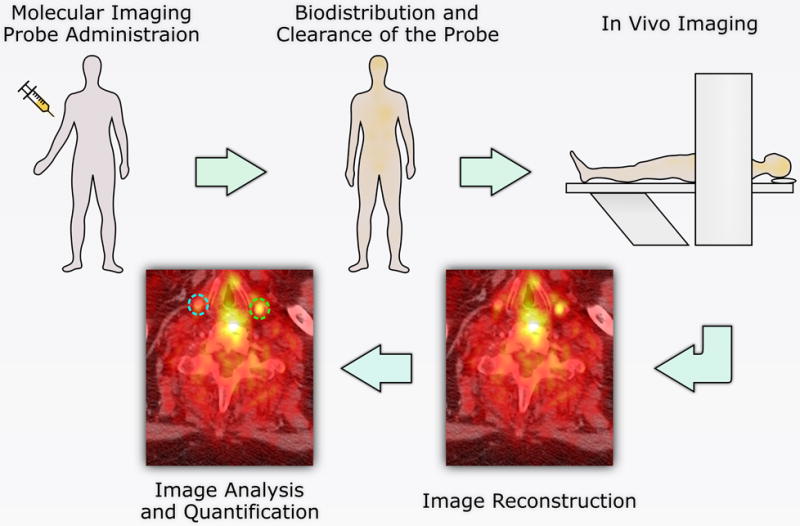
Schematic representation of molecular imaging workflow.
Different imaging modalities are used for cardiovascular molecular imaging. Many preclinical studies are based on optical, e.g., near-infrared fluorescent, imaging. A major disadvantage of this technique is the limited potential for non-invasive imaging in humans. The high sensitivity of nuclear imaging techniques (arguably the most important parameter for molecular imaging) is advantageous, especially for imaging targets expressed at low levels but the spatial resolution is relatively low. Consequently, nuclear techniques do not provide sufficient anatomical information for many applications of cardiovascular molecular imaging. Virtually all modern nuclear imaging scanners are associated with another imaging modality, typically CT. Images obtained from both modalities are coregistered to provide high resolution anatomical data facilitating interpretation of nuclear imaging data. Contrary to nuclear imaging, magnetic resonance imaging has a high spatial resolution, but is limited with regards to sensitivity. Ultrasound-based molecular imaging is relatively inexpensive, and has a reasonable temporal and spatial resolution for cardiovascular imaging but its reliance on microbubbles limits its use to mainly endothelial targets. These and other aspects of molecular imaging probes and imaging modalities will be discussed in more depth in the future issues of the journal.
Acknowledgments
Funding Sources
This work was supported by National Institutes of Health R01 HL112992, R01 HL114703, and Department of Veterans Affairs Merit Award I0-BX001750.
Footnotes
Disclosures:
None
References
- 1.Mankoff DA. A definition of molecular imaging. J Nucl Med. 2007;48:18n, 21n. [PubMed] [Google Scholar]


