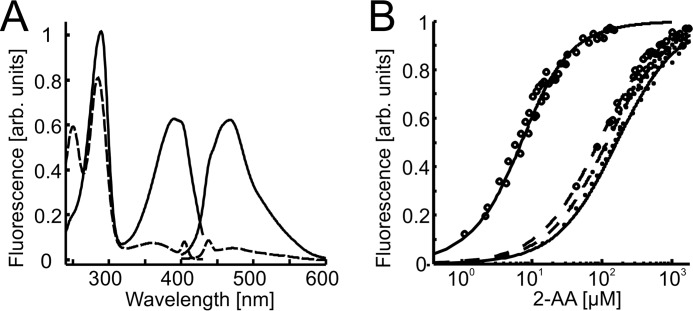
FIGURE 9.
Equilibrium binding of 2-AA to PqsBC proteins in apo- and octanoylated forms. A, fluorescence excitation and emission spectra of 2-AA (1 μm) in unbound (dashed line) and protein-bound (20 μm PqsBC, continuous line) form. B, titration data and curve fittings of 5 μm PqsBC (continuous lines) or PqsBCH269A variant (dashed lines) with 2-AA in the presence (circles) or absence (dots) of 10 μm octanoyl-CoA. Data suggest an increase of binding affinity to 2-AA of the acyl-protein in the wild-type but not in the H269A variant (numerical data provided in Table 2).