FIGURE 2.
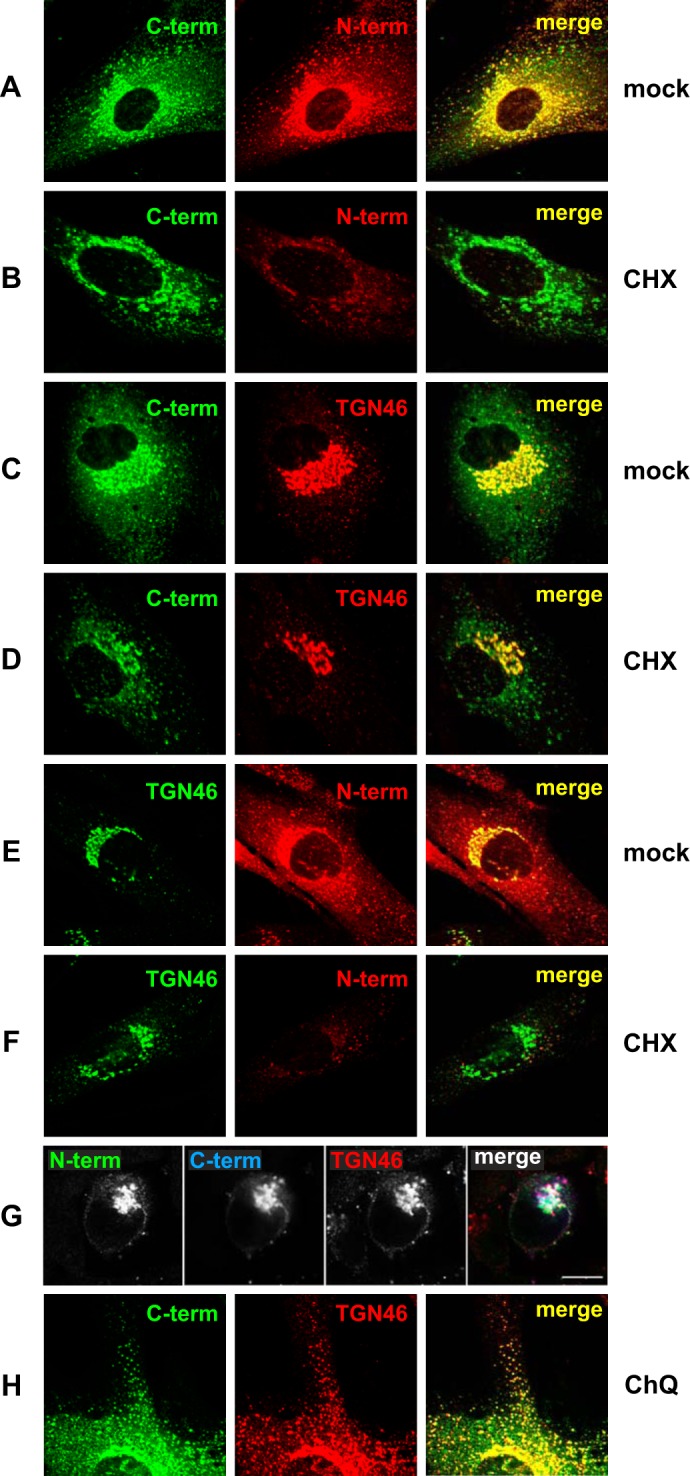
The steady state localization of E3/49K in the Golgi/TGN comprises newly synthesized uncleaved E3/49K as well as recycled C-terminal fragments that co-localize extensively with TGN46. Primary fibroblasts (SeBu) infected with Ad19a for 12 h were treated for 6 h with 50 μg/ml CHX. Subsequently, cells were processed for confocal laser microscopy by staining E3/49K with anti-49Kct (A–D) and rat mAb 4D1 against the ectodomain (N-term; A, B, E, and F). Golgi/TGN localization was determined by co-staining for the TGN marker TGN46 using either sheep polyclonal Abs (C, D, G, and H) or rabbit polyclonal Abs (E and F), followed by FITC-labeled donkey anti-rabbit IgG or rhodamine-labeled donkey anti-sheep IgG, anti-rat IgG, and anti-mouse IgG. For the triple labeling experiment (G), A549 cells infected with Ad19a for 9 h were fixed and stained with antibodies against TGN46 and the N and C termini of E3/49K. Secondary Abs were Alexa Fluor 488-labeled goat anti-rat IgG, Alexa Fluor 633-labeled goat anti-rabbit IgG, and Alexa Fluor 594-labeled donkey anti-sheep IgG. Images are single optical sections. Each channel was imaged separately under single laser illumination to prevent cross-over and overlaid using Leica confocal software. Scale bar, 10 μm. H, primary fibroblasts infected with Ad19a for 12 h were treated for 3 h with 100 μm chloroquine (CHQ) prior to staining with antibodies against TGN46 and anti-49Kct. The figure shows one typical experiment of three (A–F) and two (G and H), respectively.
