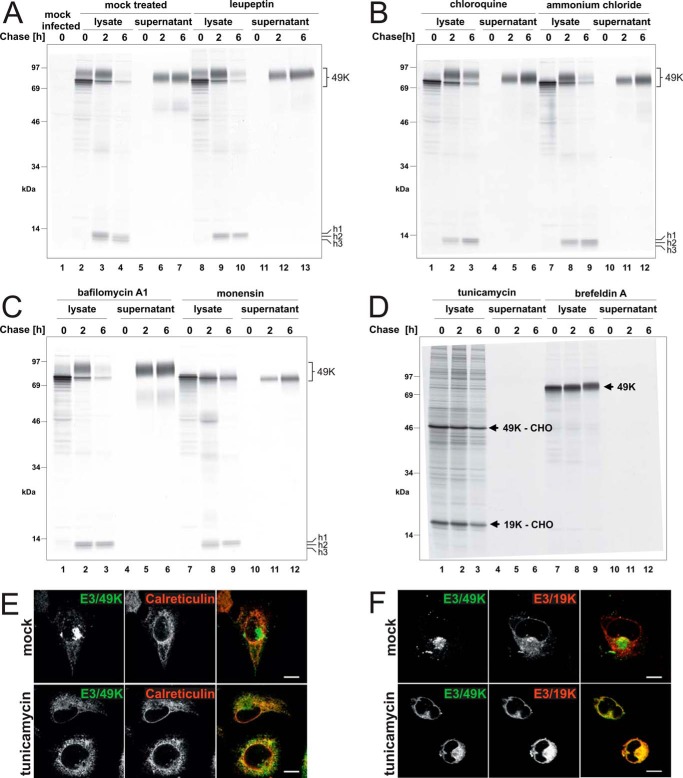
FIGURE 6.
Proteolytic processing and secretion of E3/49K in the presence of agents affecting glycosylation, endosomal/lysosomal proteases, the pH of intracellular compartments, or trafficking. Ad19a-infected A549 cells were labeled with [35S]methionine for 30 min (0 h) and chased with medium containing non-radioactive methionine for 2 and 6 h. Subsequently, detergent extracts were prepared (lysate), and E3/49K was immunoprecipitated with anti-49Kct, whereas the supernatant was processed and immunoprecipitated with anti-49K-N as described (32). The different agents indicated at the top were included both in the starvation medium and the following incubations. A, mock-treated, with the same medium changes but without inhibitors (lanes 2–7) and leupeptin (200 μm; lanes 8–13); B, chloroquine (100 μm; lanes 1–6) and ammonium chloride (10 mm; lanes 7–12); C, bafilomycin A1 (100 nm; lanes 1–6) and monensin (10 μm; lanes 7–12); D, tunicamycin (10 μg/ml; lanes 1–6) and brefeldin A (5 μg/ml; lanes 7–12). The high molecular weight E3/49K species (49K) and the C-terminal fragments h1–h3 are denoted on the right. E3/49K-CHO and E3/19K-CHO, the unglycosylated species of E3/49K and E3/19K. E, deglycosylated E3/49K colocalizes with the ER marker calreticulin upon tunicamycin treatment (3 h) in permanently transfected A549 cells expressing codon-optimized E3/49K (A549E3/49K-co15) and in infected cells (data not shown). Calreticulin was detected with a rabbit antiserum (Stressgen). F, A549 cells were infected with Ad19a for 6 h and treated for 3 h with tunicamycin (bottom panels) or were mock-treated (DMSO; top panels). Fixed cells were stained for E3/49K N terminus and the ER-resident Ad19a protein E3/19K using rabbit antiserum R22612 and imaged as in Fig. 2G. Scale bar, 20 μm. All experiments were carried out at least twice.