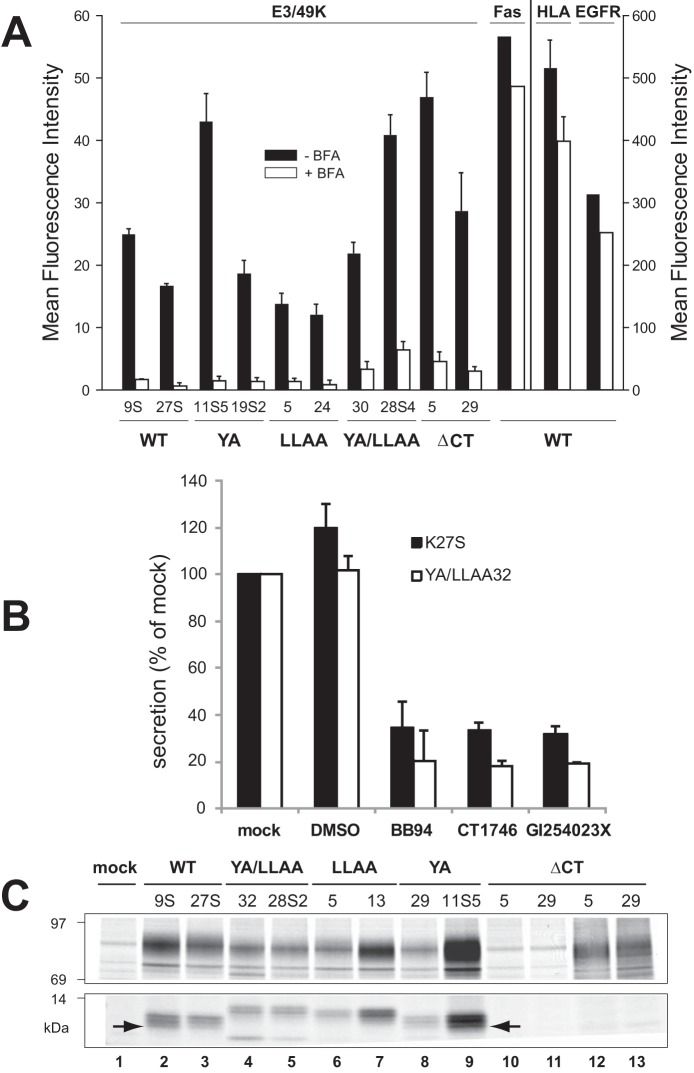
FIGURE 7.
E3/49K cleavage occurs independent of the putative transport motifs at the cell surface and is mediated by MMPs. A, the mean fluorescence intensity of E3/49K WT or mutant clones (indicated at the bottom) on the cell surface was determined by flow cytometry using mAb 4D1 after mock (black bars) and 4-h BFA treatment (5 μg/ml; white bars). Mean values and the S.D. were calculated from 4–6 independent experiments except for Fas and EGF receptor expression that was measured only once. No BFA-induced cytotoxicity or increased background staining was detectable within this time frame. B, cells expressing WT E3/49K (K27S) or the double mutant YA/LLAA (clone 32) lacking all motifs were incubated with the broad spectrum MMP inhibitors BB-94 and CT1746 and the more selective ADAM10 inhibitor GI254023X in 0.1% DMSO (final concentration) for 15–18 h. Cells were also incubated with medium alone (mock) and 0.1% DMSO as solvent control (DMSO). Supernatants were harvested and centrifuged at 1500 × g. Jurkat T cells (6 × 106) were incubated with 200 μl of supernatant, followed by detection of sec49K binding using FACS analysis. The data show the mean plus S.D. (error bars) of five independent staining experiments and three independently produced supernatants. C, mutation of the transport motifs does not prevent initial cleavage but rather secondary cleavage. Transfected clones indicated at the top expressing E3/49K WT and mutant proteins were metabolically labeled for 2 h. E3/49K was precipitated with anti-49Kct antiserum (lanes 1–11) and anti-49K-N (lanes 12 and 13). The figure shows one of two experiments. Only the relevant sections of the SDS-PAGE with the 80–100- and 10–14-kDa E3/49K species are shown. The arrow marks the C-terminal fragment h3.