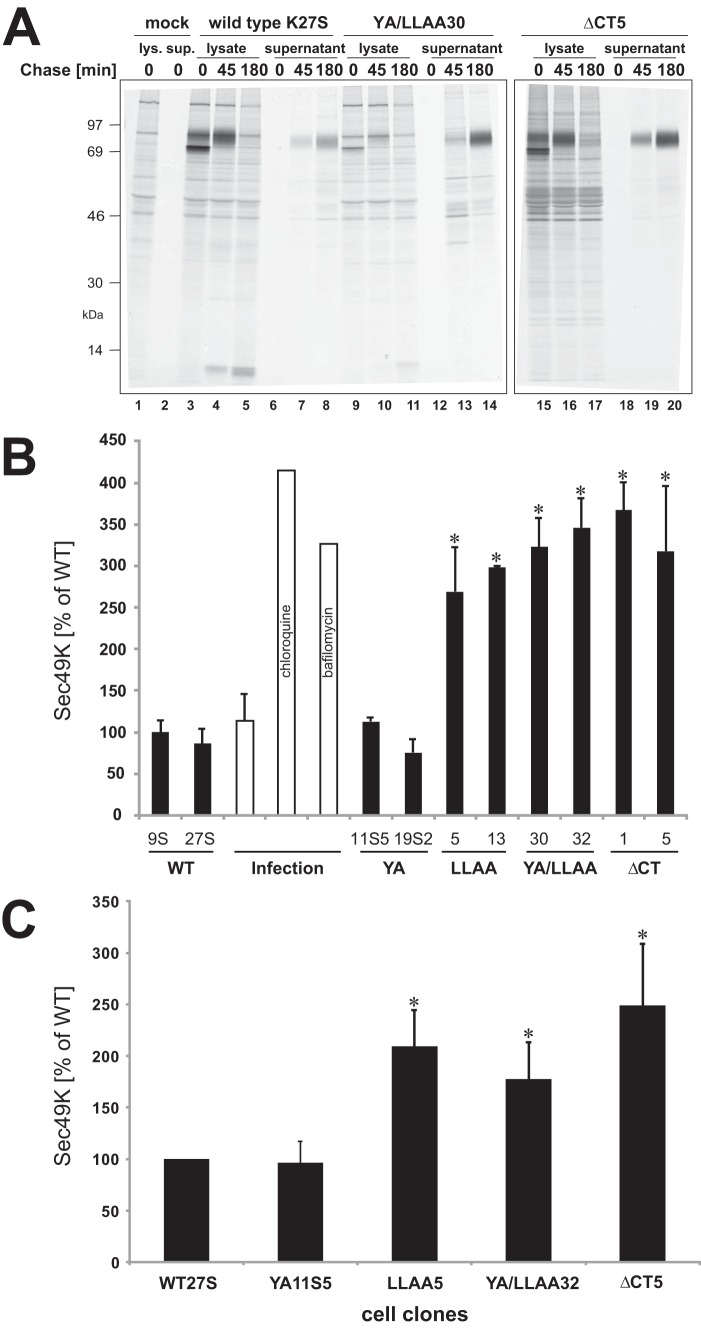
FIGURE 8.
Elimination of the LL motif alone or in combination with the YXXΦ motif enhances secretion. A, the processing kinetics of WT and mutant E3/49K proteins was determined by a 30-min pulse with [35S]methionine followed by a 45- and 180-min chase. E3/49K was precipitated from untransfected (mock), WT, and YA/LLAA mutant cell lysates with anti-49Kct (lanes 1–5 and 9–11) and anti-49K-N from ΔCT mutant cell lysates (lanes 15–17) and all supernatants (lanes 6–8, 12–14, and 18–20). B, the proportion of E3/49K secreted was quantitatively determined in a separate pulse (0.5 h)-chase (4 h) experiment followed by immunoprecipitation with anti-49K-N and SDS-PAGE. Bands corresponding to sec49K were quantified by phosphorimaging analysis and related to the radioactivity in E3/49K high molecular weight species after the pulse that was defined as 100%. The maximum amount is secreted when 80% of the total E3/49K labeling is released because the cytoplasmic tail contains 20% of the total [35S]methionine labeling. Mean values were calculated from two independent experiments except for the values obtained upon chloroquine and bafilomycin A1 treatment, which were derived from single experiments. The mean value of WT clone K9S was set as 100%. Error bars, S.D. A significant difference between mutants and WT is indicated by asterisks (p < 0.05). C, enhanced lymphocyte binding activity (sec49K) is observed in supernatants of mutants lacking the LL motif. One clone from WT and each mutant was assessed for the efficiency of secretion as measured using the Jurkat T lymphocyte binding assay. Cells were cultured for 4 days, and the supernatant was harvested and centrifuged. Three independently collected supernatants were analyzed for the presence of sec49K in at least four different experiments as described under “Experimental Procedures.” Error bars, S.D. A significant difference between mutants and WT is indicated by asterisks (p < 0.05).