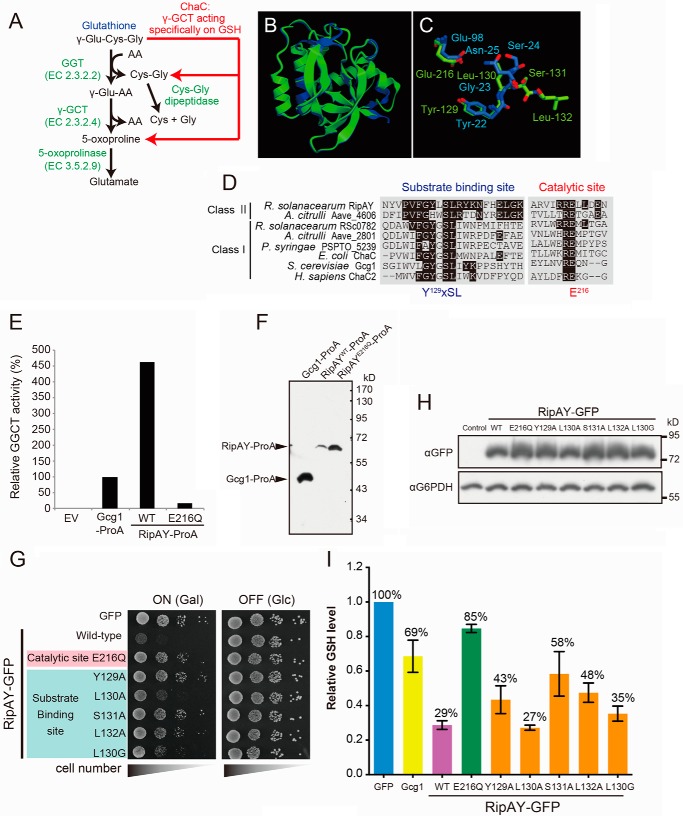
FIGURE 2.
Expression of RipAY in yeast causes growth inhibition by depletion of intracellular glutathione through its conserved ChaC domain. A, the GSH catabolism by a classical γ-glutamyl transpeptidase- and GGCT-dependent pathway and a novel ChaC family-dependent pathway. GGT, γ-glutamyl transpeptidase. B, the RipAY protein was modeled using the Phyre2 server. The homology model obtained was superimposed on the crystal structure of GGCT (C7orf24, Protein Data Bank codes 2PN7 and 2RBH) using the graphics program CueMol. Shown is superimposition of homology-modeled RipAY structure (green) on GGCT structure (blue). C, putative active site residues of RipAY (Y129LSL and Glu216; green) were superimposed on corresponding active site residues of GGCT (Y22GSN and Glu98; blue). D, multiple alignment of amino acid sequences in the regions of putative substrate binding and catalytic sites of the class I and II ChaC proteins. The conserved amino acids, Y129XSL and Glu216, are the putative substrate binding site and catalytic glutamate residue for GGCT, respectively. E, Protein A-tagged Gcg1, RipAY WT, and active site mutant, E216Q, were expressed in a glutathione-degrading enzyme-deficient strain (dug3Δ ecm38Δ gcg1Δ triple mutant) of yeast cells and immunopurified with IgG-beads. The GGCT activity of the beads immobilized with Gcg1, RipAYWT, or RipAYE216Q proteins was measured by a Dug1-coupled method as described under “Experimental Procedures.” EV, empty vector. F, the proteins extracted from the beads shown in E were resolved on SDS-PAGE followed by immunoblotting using a rabbit IgG. G, mutations in putative substrate binding and catalytic sites of RipAY restore the yeast growth inhibition caused by expression of RipAY. Yeast cells carrying empty vector or GAL1 expression vector of WT or the indicated mutant proteins of RipAY were spotted on SD (−Ura) and SGal (−Ura) plates and cultured for 2 days and 3 days, respectively. H, yeast cells carrying GAL1 expression plasmid of GFP-tagged WT or the indicated mutant proteins of RipAY were grown in SD (−Ura) liquid medium to mid-log phase and then shifted to SGal (−Ura) liquid medium to induce the protein expression, further cultured for 12 h, and analyzed by immunoblotting with anti-GFP antibody. I, mutations in putative substrate binding and catalytic sites of RipAY restore the reduction of intracellular GSH level in yeast caused by expression of RipAY. The total GSH levels of the cell lysates from yeast cells expressing the indicated proteins were measured as described under “Experimental Procedures.” Values represent the mean ± S.E. (error bars) (n ≥ 3).