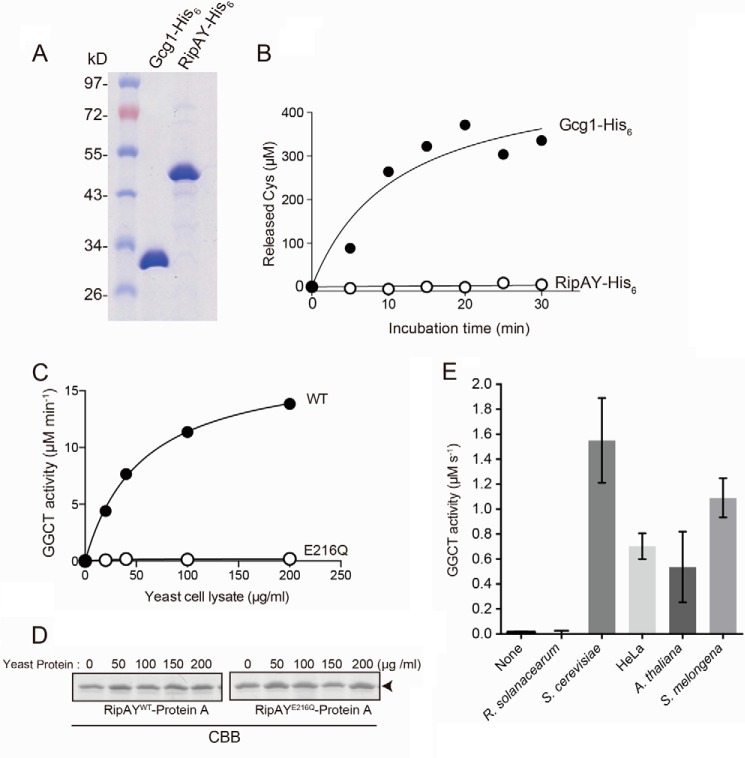
FIGURE 5.
RipAY exhibits GGCT activity in the presence of eukaryotic factor(s). A, purification of recombinant Gcg1 and RipAY proteins. Recombinant His6-tagged Gcg1 and RipAY were expressed in E. coli and purified using nickel-nitrilotriacetic acid affinity chromatography. Purified proteins were analyzed on SDS-PAGE with Coomassie Blue staining. B, measurement of GGCT activity of recombinant Gcg1 and RipAY purified from E. coli. 0.1 μg of Gcg1 and 1 μg of RipAY were incubated with 5 mm glutathione, the reactions were terminated at the indicated time points, the released Cys-Gly dipeptides were digested with Cys-Gly dipeptidase Dug1, and then the released Cys was measured by acidic ninhydrin. C, effect of concentration of the yeast protein extract on activation of GGCT activity of RipAY. The beads bound with RipAYWT or RipAYE216Q proteins expressed in E. coli were preincubated with different concentrations of native yeast protein extracted from a glutathione-degrading enzyme-deficient strain (dug3Δ ecm38Δ gcg1Δ triple mutant) for 2 h at 4 °C and washed with the same buffer three times, and then GGCT activity of the beads was measured by the Dug1-coupled method. D, the Protein A-tagged RipAYWT and RipAYE216Q proteins extracted from the beads shown in C were resolved on SDS-PAGE and visualized by Coomassie Brilliant Blue (CBB) staining. E, the GGCT activity of bacterially expressed RipAY-His6 protein incubated with 10 μg of protein of the cell lysates from R. solanacearum, S. cerevisiae, human cultured cell HeLa, A. thaliana, or eggplant S. melongena cells was measured by a Dug1-coupled method. Values represent the mean ± S.E. (error bars) (n ≥ 3).