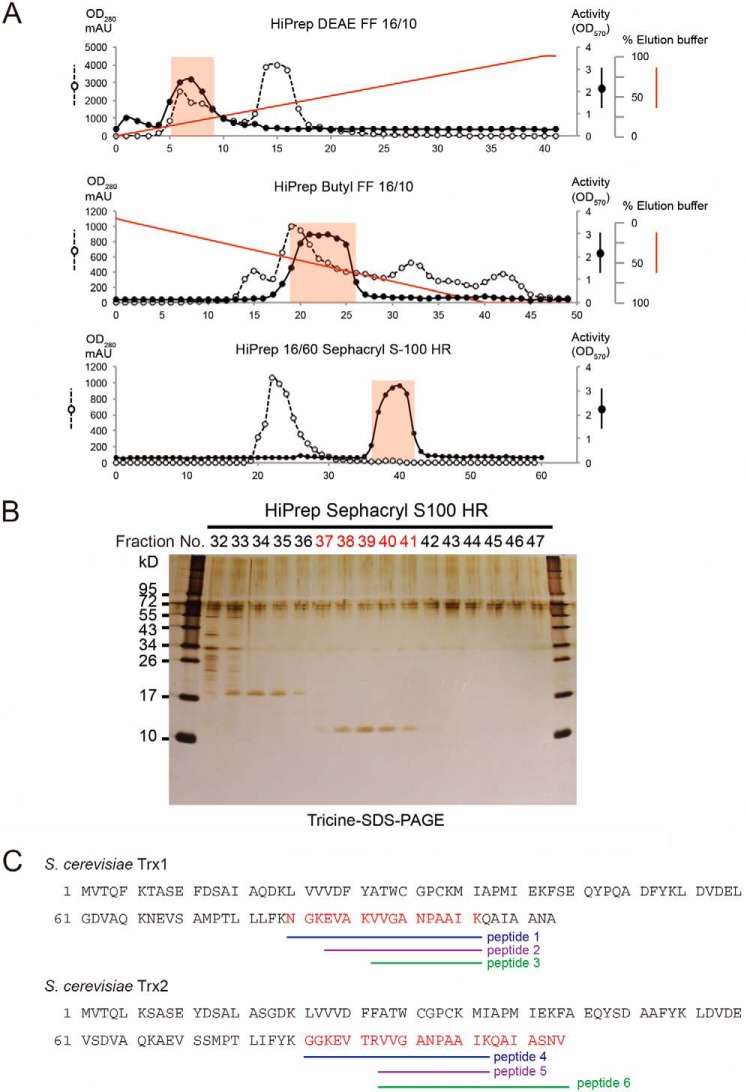
FIGURE 6.
Identification of thioredoxin as a eukaryotic activator for GGCT activity of RipAY. A, biochemical purification of the eukaryotic activator of RipAYfrom yeast protein extracts by HiPrep DEAE FF 16/10 ion exchange, HiPrep Butyl FF 16/10 hydrophobic interaction, and HiPrep 16/60 Sephacryl S-100 HR gel filtration chromatography. B, gel filtration fractions surrounding activity were loaded onto a Tricine SDS-polyacrylamide gel, visualized by silver staining. C, gel bands of fractions 37–41 stained with Coomassie Brilliant Blue were collected and in-gel digested, and following LC-MS/MS analysis, yeast thioredoxin Trx1 and Trx2 proteins were identified as the activator. Amino acid sequences of S. cerevisiae Trx1 and Trx2 and their corresponding tryptic peptides identified by LC-MS/MS analysis are shown.