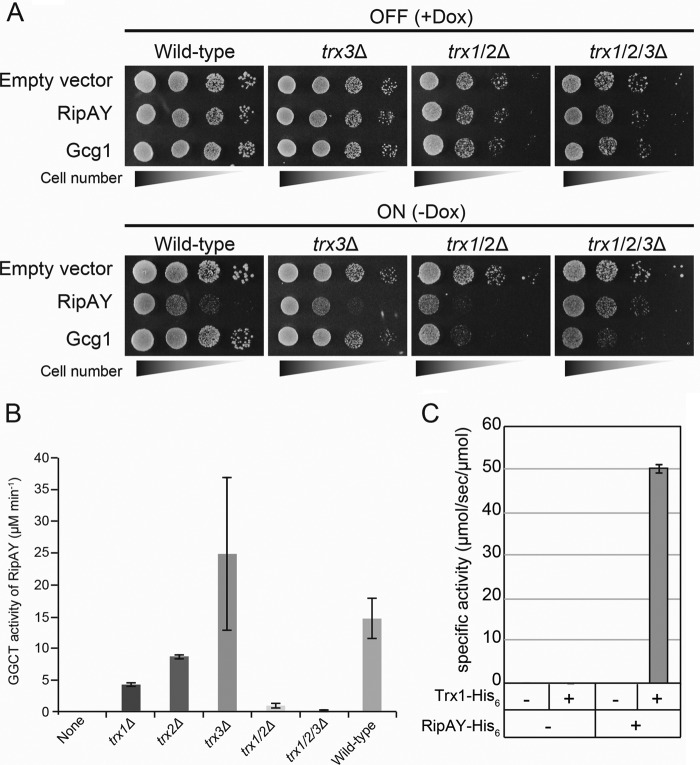
FIGURE 7.
Yeast thioredoxins can activate a GGCT activity of RipAY both in vivo and in vitro. A, S. cerevisiae WT, trx3Δ single mutant, trx1/2Δ double mutant,and trx1/2/3Δ triple mutant cells carrying plasmid expressing RipAY or Gcg1 under the control of the Tet-off promoter were grown in noninducing (+Dox) or inducing (−Dox) conditions. Yeast cells transformed with corresponding empty vector were used as a control. The cells were grown at 26 °C for 3 days for SD (−Ura, +Dox) and 4 days for SD (−Ura, −Dox). B, the GGCT activity of bacterially expressed RipAY-His6 protein incubated with 10 μg of protein of the cell lysates from the indicated S. cerevisiae thioredoxin mutant cells was measured by the Dug1-coupled method. Values represent the mean ± S.E. (error bars) (n ≥ 3). C, bacterially expressed S. cerevisiae Trx1-His6 protein can activate the GGCT activity of bacterially expressed RipAY-His6 protein.