FIGURE 4.
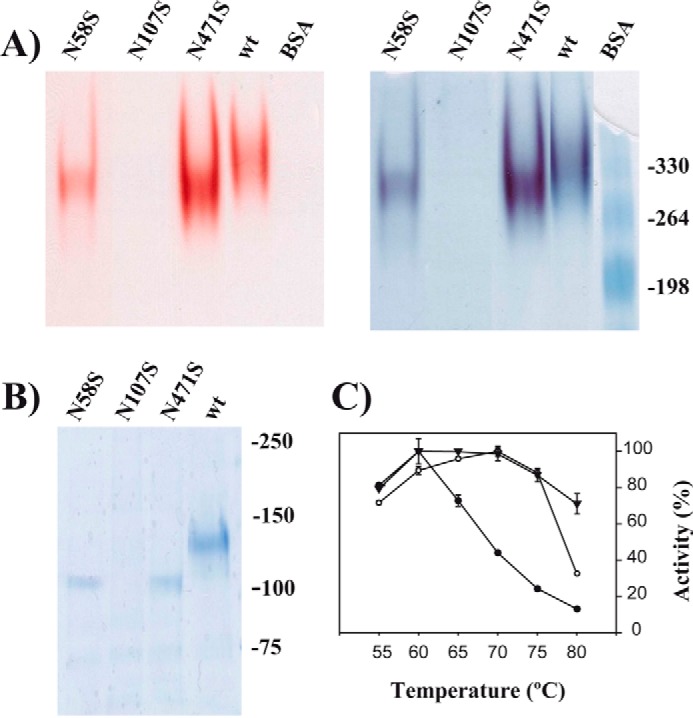
Role of glycosylation in XdINV. A, PAGE analysis of the XdINV variants. Culture filtrates (200 ml) of the P. pastoris transformants expressing the wild-type (wt) or the indicated XdINV mutants were concentrated through 50000 Mr cutoff (PES membrane) and applied to DEAE-Sephacel chromatography. Active fractions eluting at 0.1 m NaCl were concentrated to 0.2–0.5 mg/ml. The enzyme activities (10 μl; about 2–5 μg of proteins) were revealed in situ (left) and subsequently stained using colloidal Coomassie (right). The mutant N107S was processed in the same way, but 20 μl of the final concentrate was analyzed. B, referred proteins were analyzed by SDS-PAGE. Numbers at the right in A and B indicate the positions of the bovine serum albumin (BSA) molecular masses and the weight markers used as control (in kDa), respectively. C, temperature dependence profiles. Activity of the wild-type (filled triangles), N58S (filled circles), and N471S (open circles) protein variants were evaluated at the indicated temperatures. Each value represents the average for four independent measurements. Standard errors are indicated.
