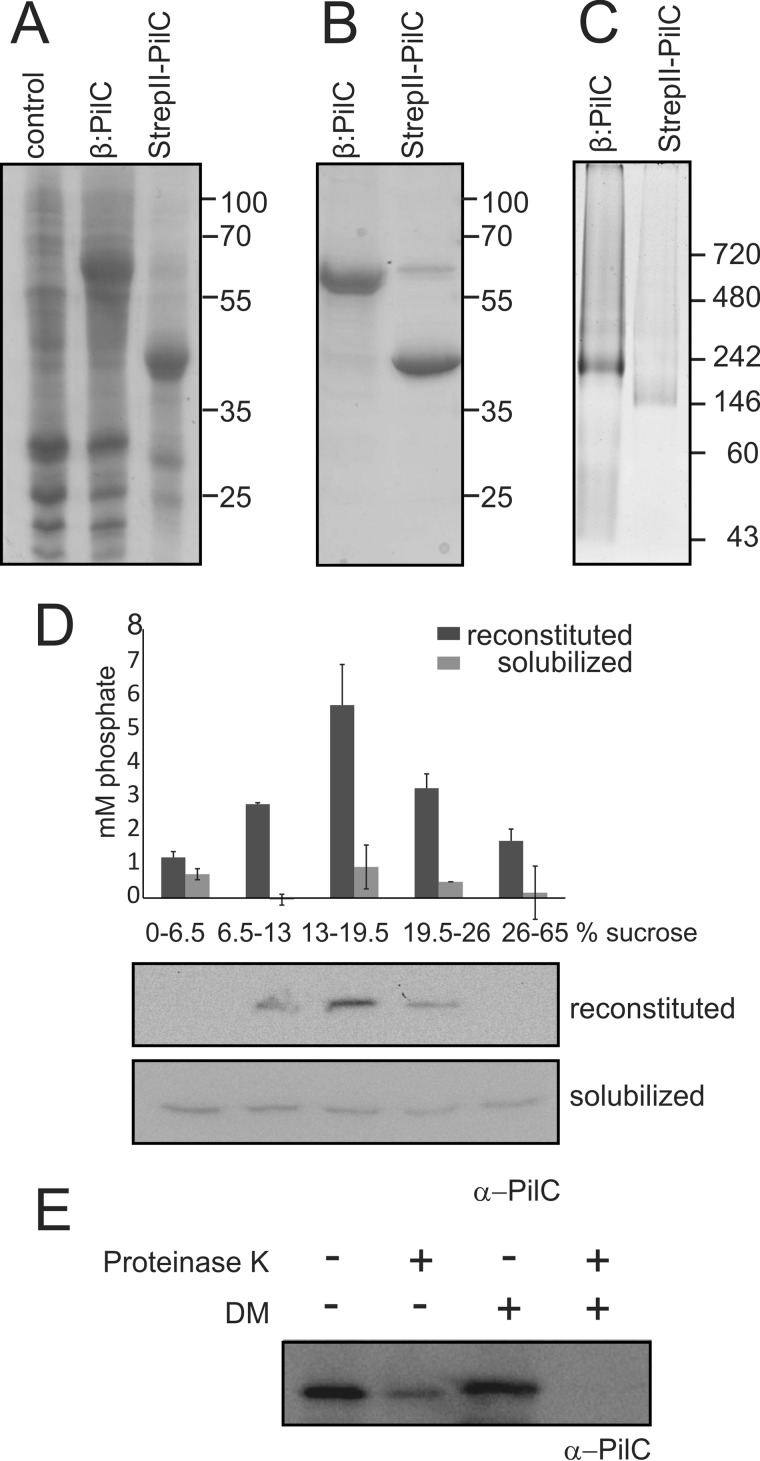
FIGURE 1.
Expression, purification, and reconstitution of PilC. A, Coomassie-stained SDS-PAGE of membranes isolated from E. coli Rosetta 2 (DE3) strains expressing no PilC (control), His6-YbeL-TEV-StrepII-PilC (β:PilC), and His6-YbeL-TEV-StrepII-PilC-expressing membranes treated with TEV protease (StrepII-PilC). Representative figure is of at least 10 biological replicates. B, Coomassie-stained SDS-PAGE of purified His6-YbeL-TEV-StrepII-PilC (β:PilC) and StrepII-PilC. Representative figure is of at least 10 biological replicates. C, Blue-Native PAGE analysis of purified His6-YbeL-TEV-StrepII-PilC (β:PilC) and StrepII-PilC. Representative figure is of three biological replicates. Non-native (A and B) and native (C) molecular mass markers are indicated. D, reconstitution of purified StrepII-PilC was analyzed by sucrose density gradient centrifugation. Solubilized and reconstituted PilCs were loaded onto a sucrose step gradient. Samples from each layer were analyzed for the amount of phospholipid-derived phosphate (upper graph) and the presence of StrepII-PilC by immunoblot analyses using α-PilC antibody (lower lanes). The indicated error bars represent the average of two biological replicates containing two technical replicates. E, orientation of PilC in proteoliposomes was analyzed by incubation of PilC-containing proteoliposomes with (+) or without (−) 1 mg/ml proteinase K in the presence (+) and absence (−) of 0.5% n-decyl-β-d-maltopyranoside (DM), followed by Western blotting using an α-PilC antibody, directed against the 185 N-terminal cytoplasmic amino acids. Representative figure is of two biological replicates with two technical replicates.