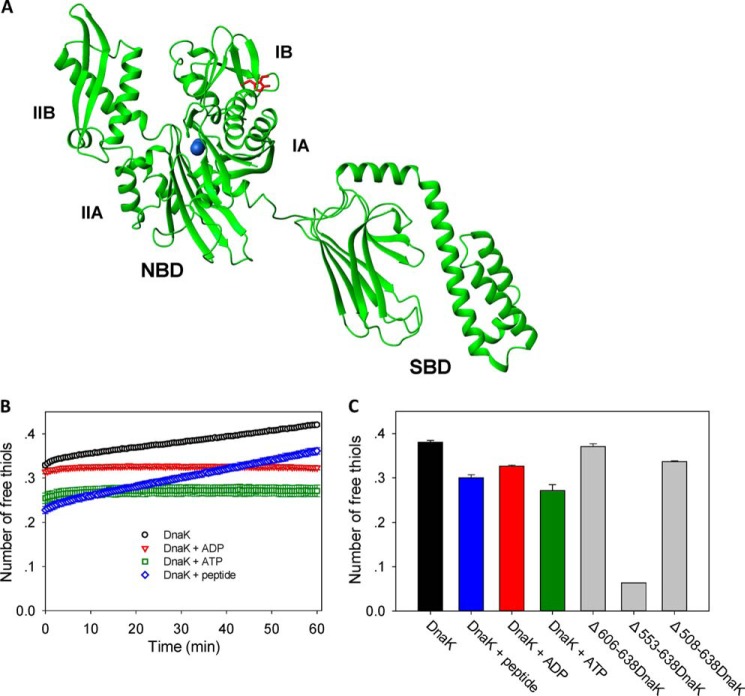
FIGURE 1.
Position and reactivity of the single cysteine residue in DnaK. A, DnaK structure and locations of single cysteine (Cys-15) and tryptophan (Trp-102) residues. The NMR structure of E. coli DnaK (Protein Data Bank code 2kho) is displayed in green, the Cys residue is in blue, and the Trp residue is in red. The N-terminal NBD and its subdomains IA, IB, IIA, and IIB and the C-terminal SBD are indicated. B, the time course of cysteine reactivity of DnaK in the absence and presence of ADP, ATP, or peptide was monitored in a Fluostar plate reader. The number of free thiols per DnaK molecule was measured by DTNB assay as an indicator of cysteine reactivity. The number of free thiols was calculated from the absorbance at 412 nm using a standard curve. The data shown are the mean of three independent measurements, and the error bars represent the S.E. C, the number of free thiols measured at the 30-min time point is plotted to allow comparison of cysteine reactivity of WT DnaK in the absence and presence of ADP, ATP, or peptide and with the truncation mutants Δ606–638DnaK, Δ553–638DnaK, and Δ508–638DnaK. The data shown are the mean of three independent measurements, and the error bars represent the S.E.