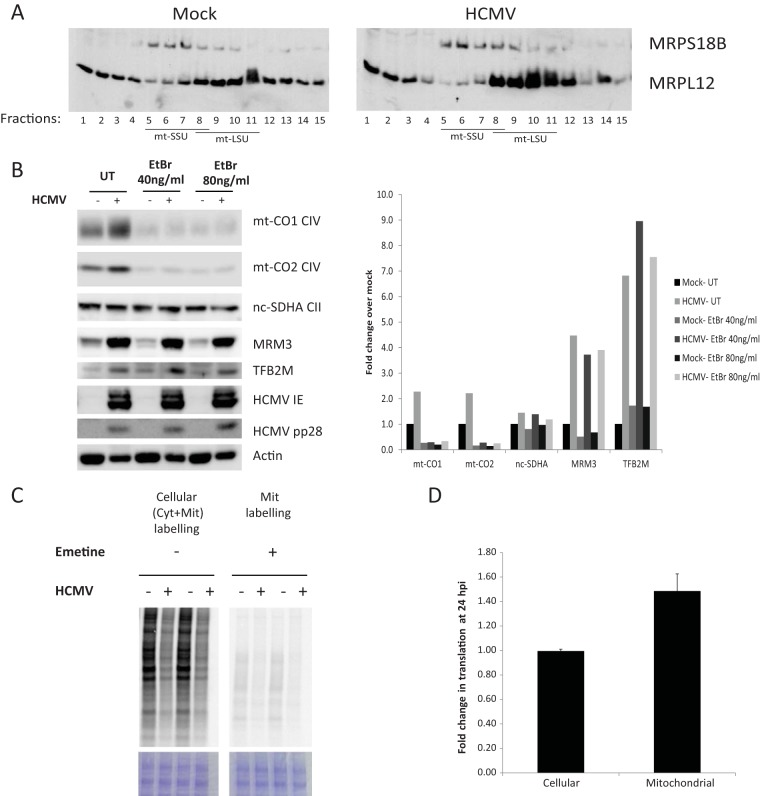
FIG 5 .
HCMV induces the synthesis and stabilization of mitochondrially encoded proteins. (A) Assembled mitoribosomes are induced by HCMV. Equal amounts of proteins of total cell lysates from mock-infected or HCMV-infected HFFF2 cells at 48 hpi were separated on a linear sucrose gradient (10 to 30% [wt/vol]) and analyzed by WB. Free nonassembled ribosomal subunits (SSU, small subunit; LSU, large subunit) migrate to the lower fractions, while assembled mitoribosomes appear in the higher-density fractions. (B) Induction of mitochondrially encoded proteins by HCMV. HFFF2 cells were either mock infected or were infected with HCMV at an MOI of 5. After 2 h, the inoculum was washed, and cells were refreshed with untreated medium (UT) or with medium containing ethidium bromide (EtBr) to block mitochondrial transcription. Cells were harvested at 48 hpi, and lysates were processed and analyzed as described in the legend to Fig. 2. The chart shows the fold change in protein abundance. HCMV induces mitochondrial translation. (C to D) Mock-infected and HCMV-infected cells were radiolabeled with [35S]methionine at 24 hpi in the presence of emetine (which blocks cytosolic translation) to determine mitochondrial translation or in the absence of emetine to determine cellular translation. Total cell lysates were separated on a 4 to 12% Bis-Tris Plus PAGE. Equal loading of the gels was confirmed by staining the gel with Coomassie brilliant blue G-250. Dried gels were exposed to a phosphorimager screen and visualized using a phosphorimager scanner (C). Radioactive counts in cell lysates were measured directly or after TCA precipitation and used to calculate cellular and mitochondrial translation efficiencies, as described in the text. The chart shows the fold change in translation after infection (D).