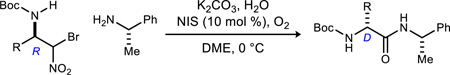
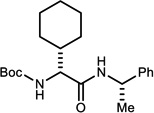
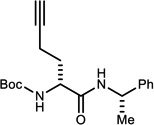
Table 3.
NIS-Catalyzed Umpolung Amide Synthesis to Pre-pare α-Amino Amides Bearing Aliphatic Substituents
Reaction run in DME, 1.2 equiv. amine for 24 h at 0 °C under an atmosphere of O2
Isolated yields.
Measured by 1H NMR. α-Bromo nitroalkanes for entries 1,3,4 were recrystallized to 99% ee (see Supporting Information). α-Bromo nitroalkanes for entries 2 and 5 were 92 and 90% ee, respectively.