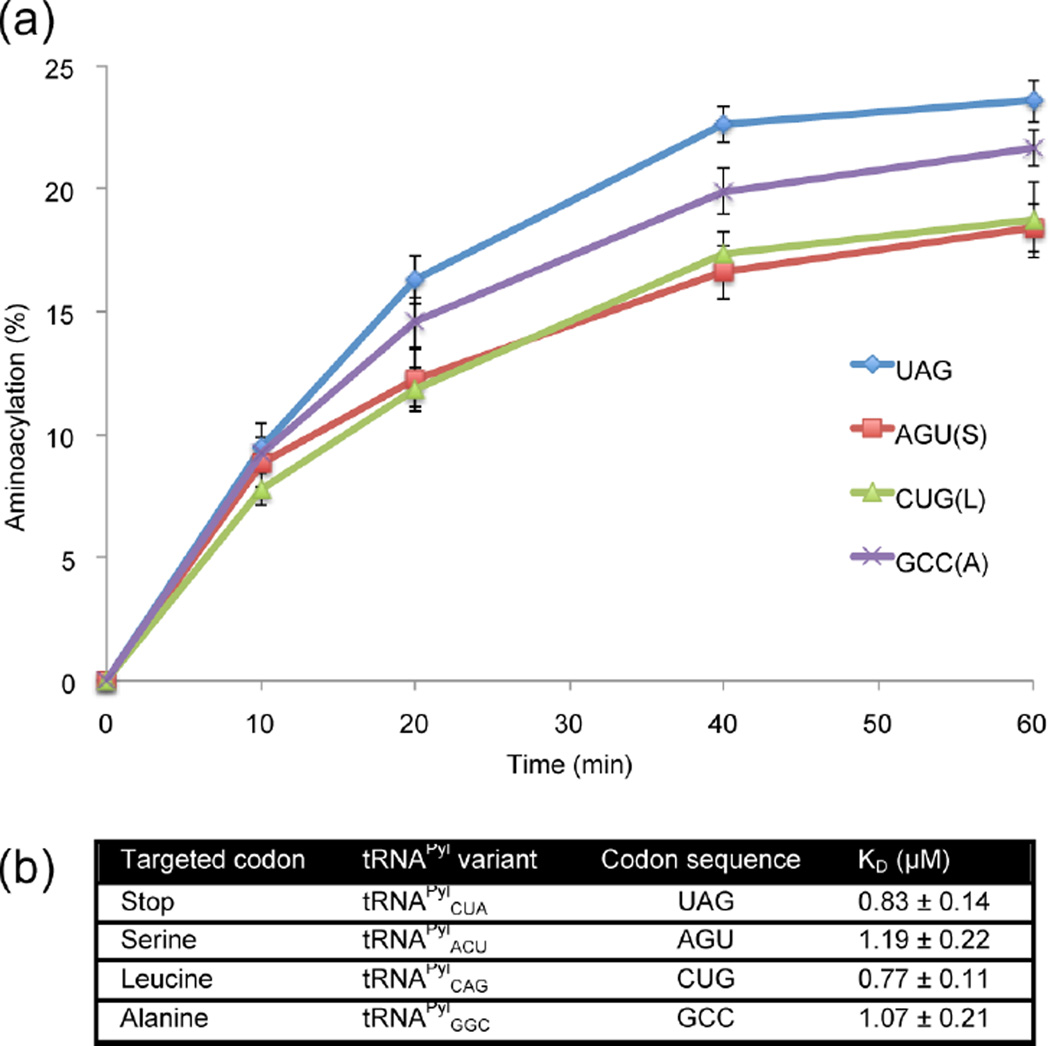
Figure 2.
Biochemical characterization of the aminoacylation efficiencies and dissociation constants of IFRS for each tRNAPyl anticodon variant. (a) The aminoacylation efficiency of IFRS for each of the anticodon variants was measured via an aminoacylation assay, where 3′-end-labeled tRNAPyl anticodon variants were incubated with IFRS and 3-I-Phe to measure the amount of product formed over 60 min at 37 °C. (b) Binding affinities were determined via filter binding assay, where varying concentrations of IFRS were incubated on ice with 3′-end-labeled tRNAPyl and the fraction of bound IFRS was measured to obtain dissociation constants.