Fig 1. Schematic representation of the TIM Barrel Fold topology.
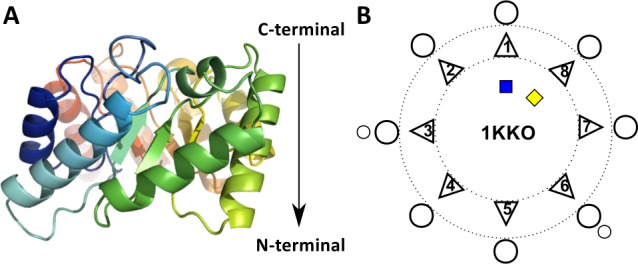
(A) A cartoon representation of a TBF domain structure, from methylaspartate ammonia lyase (PDB 1KKO). The sequence consists of 8 β-strand-α-helix repeats that form a barrel-like structure. This barrel possesses directionality in terms of a C-terminal end, represented at the top of the structure here and the N-terminal end at the bottom, indicated by the arrow. (B) The two-dimensional secondary structure element arrangement as viewed from the top of the C-terminal end of the enzyme. The diversification of the fold occurs with the addition of secondary structure elements, typically at the C-terminal end, indicated here by small circles. The triangles represent the β-strands, the circles represent the α-helices, and the blue and yellow squares are the N- and C-termini respectively. The dotted lines represent the circular spatial arrangement of the TBF, such that the β-strands are able to close and form a parallel β-barrel core, flanked by their α-helices.
