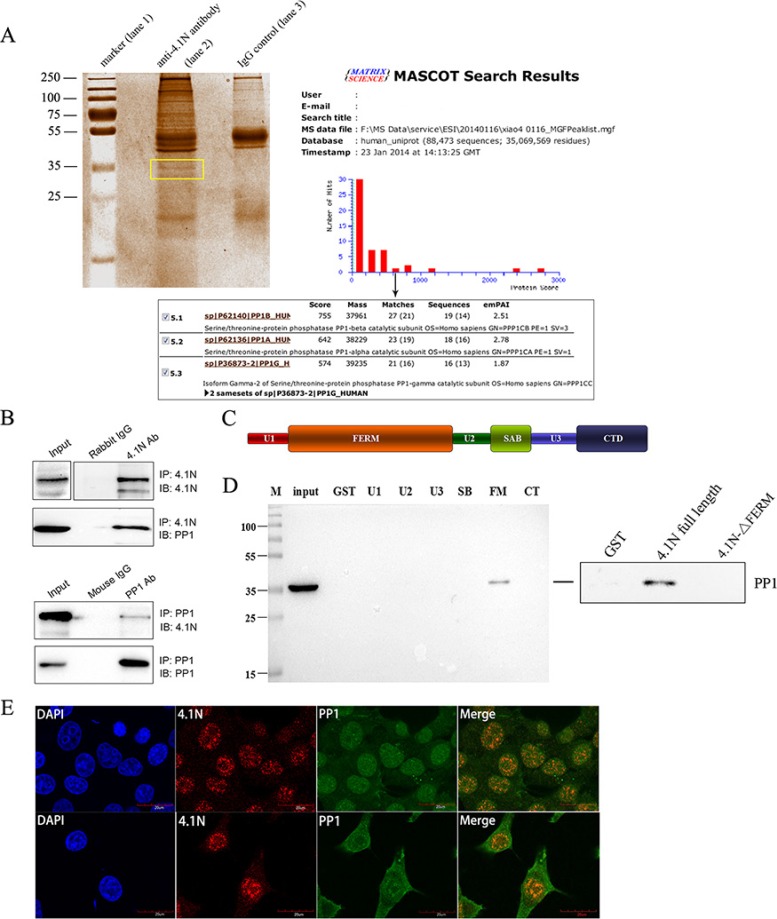
Figure 4. Interaction of 4.1N and PP1 in 95C cells.
(A) Coomassie blue-stained gel of immunoprecipitated proteins. Protein precipitates were immunoprecipitated with an anti-4.1N antibody (lane 2) and with the IgG negative control (lane 3). The differentially expressed band is indicated by the frame. The PP1 catalytic subunit, PP1 (α, β and γ2) was identified by the MASCOT search engine used to assess the data from LC-MS/MS by the identification of proteins from the Uniprot protein database. PP1 had the fifth-highest hit score among all possible hits that contained the sequences that matched the peptides within the sample. (B) 4.1N was immunoprecipitated from 95C cell lysates with an anti-4.1N antibody. 4.1N or PP1 in the immunoprecipitate was detected with an anti-4.1N or an anti-PP1 antibody. In contrast, PP1 was immunoprecipitated from 95C cell lysates with an anti-PP1 antibody. PP1 or 4.1N in the immunoprecipitate was detected with an anti-PP1 or an anti-4.1N antibody. (C) Schematic diagram showing the 4.1N protein domain organisation. 4.1N is composed of N-terminal FERM domain, an internal spectrin-actin-binding domain (SABD) and a C-terminal domain (CTD), which are separated by three unique regions (U1, U2 and U3). (D) Extracts from 95C cells were subjected to a pull-down assay with equivalent amounts of the GST and indicated GST-4.1N domain fusion proteins. PP1 binding was detected by immunoblotting with an anti-PP1 antibody. The endogenous PP1 in the extract was used as input. Right panel: Binding of PP1 to GST-tagged 4.1N full length proteins or GST-tagged 4.1N-ΔFERM fragments. Binding was assessed by pull-down assay, using anti-PP1 antibody for detection. (E) Subcellular co-localization of 4.1N and PP1 in 95C cells. Cells were co-stained for 4.1N (red) and PP1 (green). Nuclei were stained with DAPI (blue). The yellow/orange in the merged image indicates the co-localization of 4.1N and PP1. Scale bars: 20 μm.