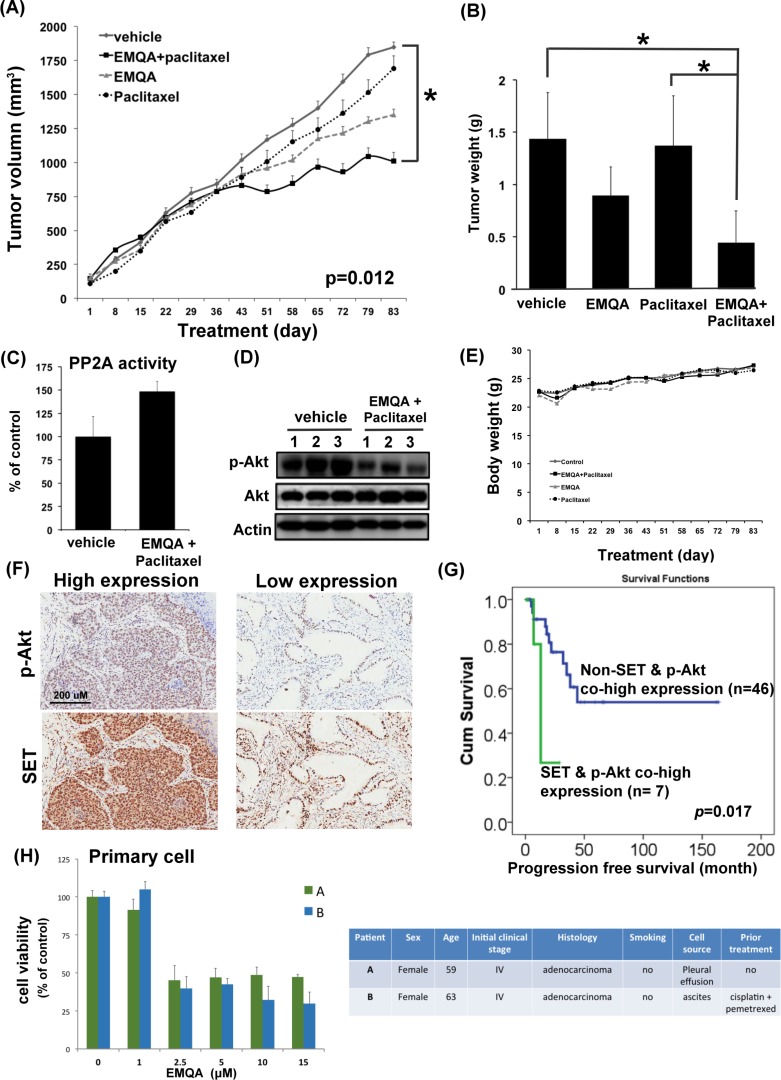
Figure 8. The in vivo effects and clinical relevance on targeting SET/PP2A/p-Akt for the treatment of NSCLC.
(A) The growth curves of A549 xenografted tumor in nude mice treated with vehicle, paclitaxel 3 mg/kg twice a week and/or EMQA 5 mg/kg/day. Points, mean; Bar, S.E. *P <0.05 (n = 10 in each treatment arm) (B) The average tumor weight of resected A549 xenografted tumor after exposing to vehicle, paclitaxel and/or EMQA treatments measured at the end of experiments. Bar, mean; error bar, S.D. (C) Analysis of PP2A activity of A549 xenografted tumor in vehicle- and EMQA and paclitaxel- treated nude mice. Bar, mean; error bar, S.D. (n = 10) (D) Representative western blot image of the expression of p-Akt and Akt in A549 xenografted tumor lysate. (E) The average body weights of nude mice exposed to the indicated treatments. (F) The expression level of SET and p-Akt were significantly correlated in the tumors. Representative images of the immunohistochemical staining of SET and p-Akt in tumors obtained form identical patient. (G) Co-high expression of SET and p-Akt is a poor prognosis factor in NSCLC patients. Progression-free survival from the time of diagnosis in NSCLC patients with and without co-expression of SET and p-Akt was compared by log-rank test. (H) EMQA induced cell growth inhibition on primary malignant cells obtained from two NSCLC patients. Viabilities of primary cells gathered from patient A and B after exposure to EMQA at indicated doses for 72 hours were assessed by MTT (left panel). Bar: mean, error bar: S.D. The basic characteristics of patient A and B were summarized at right panel.