Abstract
We measured pupillary constrictions in response to full-screen flashes of variable luminance, occurring either at the onset of a saccadic eye movement or well before/after it. A large fraction of perisaccadic flashes were undetectable to the subjects, consistent with saccadic suppression of visual sensitivity. Likewise, pupillary responses to perisaccadic flashes were strongly suppressed. However, the two phenomena appear to be dissociable. Across subjects and luminance levels of the flash stimulus, there were cases in which conscious perception of the flash was completely depleted yet the pupillary response was clearly present, as well as cases in which the opposite occurred. On one hand, the fact that pupillary light responses are subject to saccadic suppression reinforces evidence that this is not a simple reflex but depends on the integration of retinal illumination with complex “extraretinal” cues. On the other hand, the relative independence of pupillary and perceptual responses suggests that suppression acts separately on these systems—consistent with the idea of multiple visual pathways that are differentially affected by saccades.
Keywords: saccadic eye movements, pupil, perisaccadic suppression, pupillary light reflex, parallel visual pathways, vision for action
saccades are rapid ballistic eye movements. While allowing for rapidly directing our high-resolution fovea to different objects of interest, they impose heavy costs on the visual system. These include the smearing and sudden displacement of retinal images. Many processes contribute to elimination of these disturbances; one of these is a transient suppression of visual sensitivity to low-frequency luminance modulations (which can attenuate the disruptive motion signals produced by the rotation of the eyes; Ross et al. 2001; Wurtz et al. 2011). There is no consensus on the neural substrates of this suppression, but most agree that it spares the retina; it might be produced by a corollary discharge or copy of the oculomotor command, interacting with visual signals as early as in the thalamus (Burr et al. 1994; Wurtz 2008). In contrast with an early suppression site, however, there is evidence that suppression differentially affects conscious vision and unconscious visual processing (Watson and Krekelberg 2009)—visual stimuli that are completely suppressed from conscious perception may still affect subsequently presented images, creating a “shape contrast illusion.” This fits with the notion that visual processing involves multiple pathways, relatively independent of each other (Goodale and Milner 1992; Mishkin et al. 1983). This idea remains controversial despite numerous investigations; among these there is specific evidence that saccades have different effects on those supporting conscious vision and the others, e.g., pathways related to action planning (Burr et al. 2001).
Here we aimed to test for such dissociation by simultaneously measuring the effects of saccadic suppression on two kinds of responses to retinal stimulation: a perceptual response (the conscious detection of a light flash) and an automatic involuntary response (the pupillary constriction evoked by the flash).
Pupillary constriction in response to light is often thought of as a reflex behavior, supported by a mesencephalic circuit, directly fed by retinal signals (Gamlin and Clarke 1995; Loewenfeld 1993). However, there is growing evidence that this response in fact integrates complex information and depends on relatively high-level visual processing (for review see Binda and Murray 2015a). Granted that the major determinant of pupil diameter is light (Loewenfeld 1993), it has been shown that subtle pupillary constrictions can be evoked by stimuli that do not alter the level of retinal illumination, e.g., by changes of perceived brightness [during binocular rivalry (Barany and Hallden 1948; Richards 1966) or with brightness illusions (Laeng and Endestad 2012)] and even by simply evoking the idea of brightness [e.g., pictures of the sun (Binda et al. 2013b; Naber and Nakayama 2013) or mental imagery of bright scenes (Laeng and Sulutvedt 2014)]. Moreover, shifting attention to a brighter region (Binda et al. 2013a; Mathôt et al. 2013, 2014; Naber et al. 2013) or feature (Binda et al. 2014) is sufficient to induce pupillary constriction, and the pupillary response to a luminance increment is enhanced when the stimulus is made behaviorally relevant (Binda and Murray 2015b).
These results strongly suggest that a brightness signal, relatively independent of retinal illumination, participates in the specification of the pupillary light response. Is this signal subject to the effect of saccadic suppression, like the luminance signal supporting conscious perception is? Work from the 1960s indicates that saccadic suppression does affect pupillary light responses (Lorber et al. 1965; Zuber et al. 1966). These experiments showed that the pupillary constriction evoked by a briefly presented flash is substantially reduced when the flash occurs just before or during a saccade, i.e., when conscious detection of the stimulus is impaired. Interestingly, the data are suggestive of a differential effect of saccades on pupillary and perceptual responses: the suppression of pupillary responses extends over a much longer temporal window than the perceptual suppression. However, this difference of temporal dynamics alone could simply be put down to the slow temporal dynamics of the pupillary response (Barbur 2004)—the same extraretinal signal will give rise to a longer-lasting suppression when affecting a process with longer integration times, as modeled in Diamond et al. (2000). To more directly test for a dissociation between suppressive effects on the pupillary response and conscious detection, here we reexamined the work by Lorber and collaborators in conditions optimized for testing the relationship between the two phenomena: measuring both phenomena while varying the luminance of the flash about the subjective visibility threshold. This allows us to correlate pupillary and perceptual responses, obtaining a quantitative index of their interdependence.
METHODS
Subjects.
Fourteen subjects (5 women, 9 men; mean ± SD age: 24.57 ± 2.06 yr) participated in the study. All reported normal or corrected-to-normal vision. Experimental procedures were approved by the local ethics committee and were in accordance with the Declaration of Helsinki; participants gave their written informed consent.
Apparatus.
The experiment was performed in a quiet, dark room. Subjects sat in front of a monitor screen (40 × 30 cm) at a distance of 57 cm, with their head stabilized by a chin rest. Viewing was binocular. Stimuli were generated with the PsychoPhysics Toolbox routines (Brainard 1997; Pelli 1997) for MATLAB (MATLAB r2010a, The MathWorks) and presented on a CRT monitor (Barco Calibrator) with a resolution of 1,024 × 768 pixels and a refresh rate of 120 Hz, driven by a Mac Pro 4.1. Two-dimensional eye position and pupil diameter were monitored at 1,000 Hz with an EyeLink 1000 system (SR Research) with an infrared camera mounted below the screen and recording from the left eye. Pupil diameter measures were transformed from pixels to millimeters with an artificial 4-mm pupil, positioned at the approximate location of the subjects' left eye. Eye position recordings were linearized by means of a standard 13-point calibration routine performed at the beginning of each session. Synchronization between eye recordings and visual presentations was ensured by the Eyelink toolbox for MATLAB (Cornelissen et al. 2002).
Stimuli and procedure.
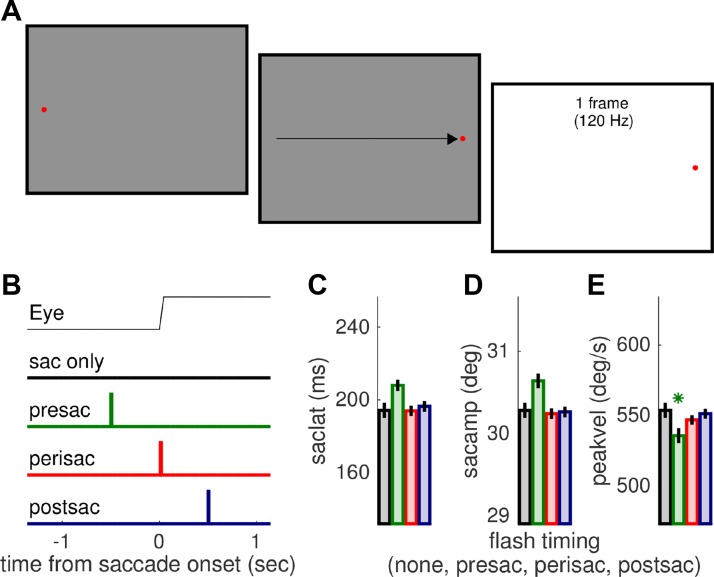
Trial structure was simple (Fig. 1A), encompassing a fixation point (displaced to elicit a saccade) and a full-screen flash (presented at variable times around the saccade). Specifically, trials began with participants fixating a red dot (0.15° across) shown on the left side of the screen (−16° of eccentricity from screen center) against a gray background (luminance of 37.2 cd/m2). After a variable delay of 1,500 ± 100 ms, the fixation point disappeared and a similar dot appeared at the opposite side of the screen (+16° of eccentricity from the center of the screen). Subjects made a saccade to the rightmost dot (the saccade target) as quickly and precisely as they could. After the saccade, gaze was to be maintained on the saccade target until the end of the trial, which had an overall duration of 4 s; an intertrial interval (ITI) of variable duration was marked by the appearance of the mouse cursor (see below). Subjects were asked to refrain from blinking at all times except during the ITI. Except in “catch” trials (15% of all trials), a full-field flash was presented for one monitor frame. The flash could take one of five possible luminance values: 62, 68, 73, 82, or 88 cd/m2. The latter was the maximum attainable luminance. Flash presentation could immediately follow the detection of saccade onset (calculated online as the first of 2 consecutive time points where horizontal eye velocity exceeded 100°/s), or it could be delayed by 500 ms relative to it. Alternatively, the flash could be shown before the saccade—its presentation time defined a priori based on the subject's saccade latency (median across all the previous trials) and an average intended delay of −500 ms. In a two-alternative forced-choice (2AFC) yes/no task, subjects reported whether they had or had not seen a flash. They did so by clicking on the top or bottom half of the screen with the mouse cursor. Collection of the response triggered the beginning of the following trial.
Fig. 1.
Methods. A: stimulus and task. Subjects made saccades from the fixation point to the saccade target (red points), as illustrated by the arrow (not part of the display). The flash stimulus was a full-field luminance increment, lasting 1 monitor frame. B: timing of the flash relative to the saccade. Except in catch trials where it was not presented, the flash could occur perisaccadically (immediately upon online saccade onset detection), 500 ms postsaccadically, or ∼500 ms presaccadically. C–E: saccade parameters (latency, amplitude and peak velocity) in the 4 conditions, averaged across trials and subjects. Presaccadic flashes tend to interfere with saccade planning, resulting in slightly delayed and larger saccades with significantly lower peak velocity, but saccade parameters were all well matched across the other conditions. Asterisk marks only significant difference (P < 0.05) between no-flash condition (black) and other conditions (color-coded as in B).
The experiment was run in two sessions, on different days. One session was completed by 10 participants and comprised the presentation of three luminance levels (68, 73, or 82 cd/m2) at three delays of flash presentation from the saccade (presaccadic, perisaccadic, or postsaccadic). Each run consisted of a randomized presentation of three trials per condition (3 repetitions × 3 contrast levels × 3 delays) plus three control trials with no flash presentation, for a total of 30 trials. The other session was completed by all subjects and comprised the presentation of two luminance levels (62 or 88 cd/m2) in the perisaccadic or postsaccadic time window. Each run consisted of a randomized presentation of six trials for each condition (6 repetitions × 2 contrast levels × 2 delays) plus six trials with no flash presentation, for a total of 30 trials. Each session lasted ∼1 h, which allowed for a maximum of eight runs (240 trials).
Analysis.
An off-line analysis examined the Eyelink output to exclude trials in which one of the following conditions was met: 1) no saccade could be detected (mean ± SE across subjects: 4.4 ± 4%), 2) saccade latency was negative (13.2 ± 4%), 3) saccade amplitude was smaller than 24°, i.e., 3/4 of the required amplitude (1.5 ± 0%), 4) a blink occurred in the interval [−1:2] s around saccade onset (10.5 ± 4%). The application of these criteria led to the inclusion of a total of 4,092 trials, corresponding to 72.9 ± 7% trials on average, with considerable variability across subjects.
The off-line analysis confirmed that pre-, peri-, and postsaccadic flashes were presented in the intended time windows: −506.32 ± 5.18, 11.99 ± 0.35, and 512.31 ± 0.32 ms from the saccade onset, respectively. For each valid trial, we studied the time course of pupil diameter in the [−1:2]-s interval around saccade onset, averaging samples into 10-ms-long bins and then subtracting the average pupil diameter in the first 500 ms of this interval. Finally, we took the minimum of each trace as an estimate of the peak pupillary response to the flash (or the peak saccade-related modulation in the catch trials with no flash) to be compared across conditions. The ultimate goal was to test whether pupillary responses to light flashes presented during the saccade are suppressed compared with postsaccadic or presaccadic flashes; for this purpose it is important to realize that our pupil recordings reflect the combination of two influences: the pupillary light response evoked by the flash and the pupillary constriction that accompanies the execution of the saccade. Because the rules governing this combination are currently unknown, we analyzed the data according to two extreme hypotheses: 1) strong subadditivity, where pupil size reflects only the largest component, and 2) perfect additivity, where the two components add up linearly. Previous work on perisaccadic pupillary responses (Lorber et al. 1965) followed the latter assumption (hypothesis 2) and estimated the light response by subtracting from each trace the average pupil modulation observed in trials with no flash presentation, which implies assuming that they are independent and additively combined. We followed this approach in our main analyses, shown in Fig. 2B, Fig. 3, B and D, Fig. 4, and Fig. 5. Subtracting the no-flash trace from the response to perisaccadic flashes will underestimate the light response if the independence between light and saccade-related pupillary constrictions is not perfect—for example, if the light response inhibits the saccade-related modulation. One extreme example of such subadditivity is described by hypothesis 1 above, in which the light response completely inhibits the saccade-related modulation. This implies that the latter must not be subtracted from the traces, but responses to peri- and pre/postsaccadic flashes must be directly compared. This approach was taken to run additional data analyses (shown in Fig. 2A and Fig. 3A). Opposite to the approach described above, this procedure is biased toward overestimating the perisaccadic light response; thus, together, the two approaches estimate the upper and lower limits of the perisaccadic light response, and consequently of the saccadic suppression effect.
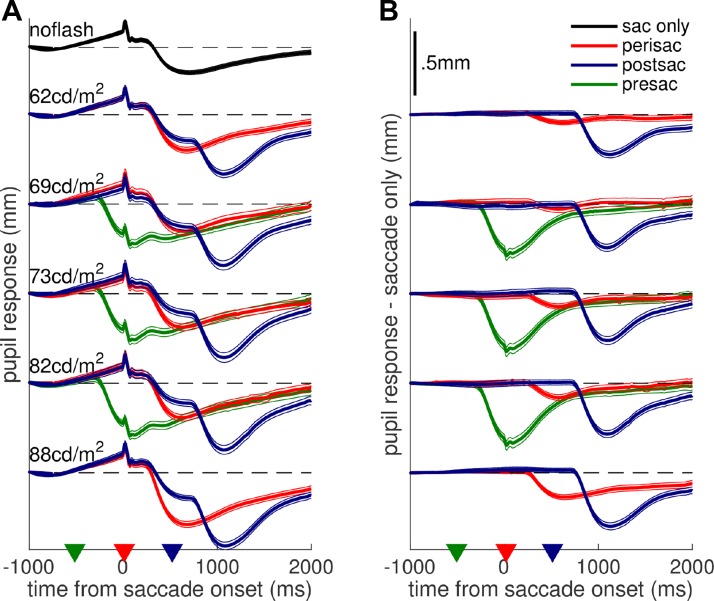
Fig. 2.
Pupillary traces. Pupil size change as a function of time from saccade onset, plotted separately for trials in which the flash occurred before/during/after the saccade or was withheld (different colors) and for the different luminance levels of the flash (y-offset; luminance as shown). Traces are averages across all trials from all subjects (with thin lines giving 95% confidence intervals), computed after subtracting from each trial the mean pupil size in the first 500 ms (A) and subtracting the average pupil trace in the saccade-only condition from each subject and experimental session (B). Black dashed lines mark 0 for each group of traces; triangles in x-axis mark the time of flash presentation. Scale is the same in A and B (shown in B, top).
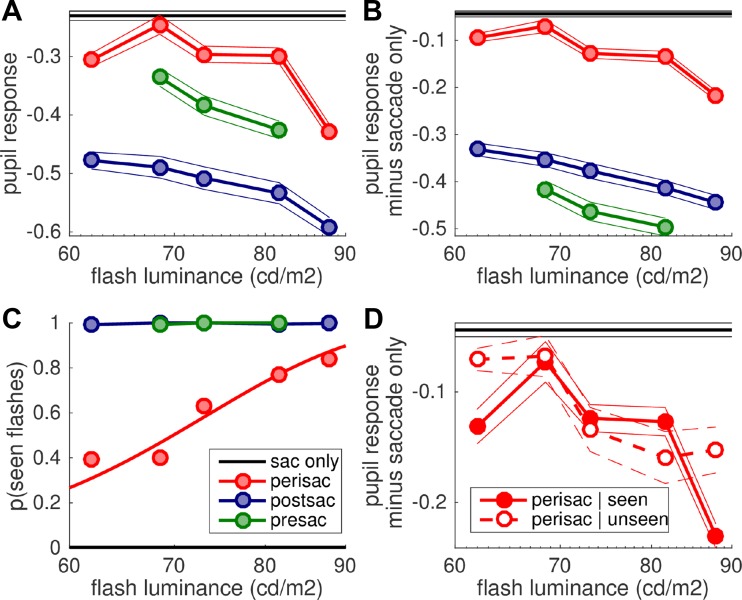
Fig. 3.
Suppression of light responses. A and B: peak pupil response (i.e., minimum of pupil traces in Fig. 2, A and B, respectively) as a function of flash luminance, with black lines giving the response in the saccade-only (no flash) condition. Symbols and thick lines give the grand average across trials from all subjects, and thin lines give 95% confidence intervals. C: proportion of trials where the flash was reported as seen. The line gives the best-fit cumulative Gaussian function across the aggregate data from all subjects (symbols). See Fig. 4 for individual psychometric functions. D: pupillary response (same conventions as in B), computed separately for perisaccadic flashes that were reported as seen or as unseen.
Fig. 4.
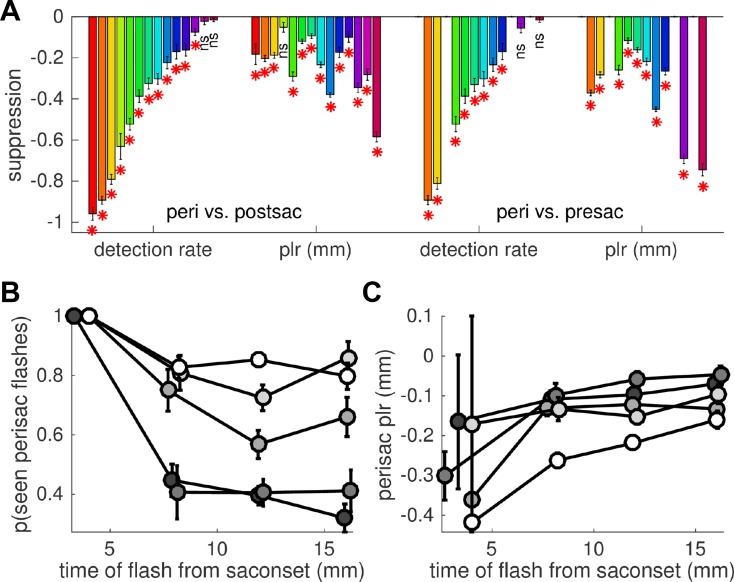
Suppression of individual light responses. A: individual suppression indexes, computed as the difference between the detection rate or the pupillary response observed for perisaccadic flashes and the same responses for postsaccadic (left) or presaccadic (right) flashes. Subjects are ordered based on the suppression of detection rate; the same order and color coding is used in Fig. 5. Error bars are SE of the difference, computed from the SE of the means of the 2 conditions considering the propagation of errors. *P < 0.05; ns, nonsignificant. B and C: average detection rate (B) and pupillary response (C) for perisaccadic flashes, plotted as a function of the exact flash time relative to the saccade onset (means in continuous nonoverlapping 5-ms bins) and shown separately for the different flash luminance levels (grayscale: highest luminance in white and lowest in dark gray). Error bars are SE; data pooled across subjects.
Fig. 5.
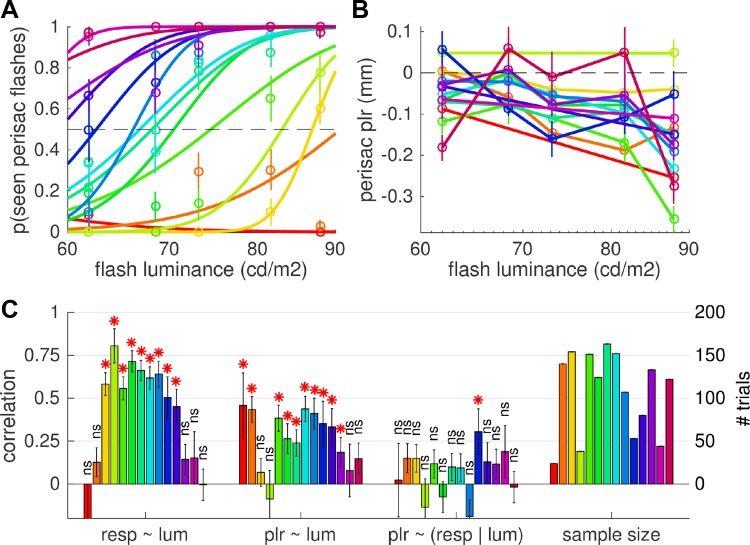
Responses to perisaccadic seen and unseen flashes. A: individual psychometric curves plotting, for each subject (color-coded, preserved across the 3 panels), the proportion of seen perisaccadic flashes against their luminance (symbols with error bars showing SE across trials) and the best-fit cumulative Gaussian function across the data. B: pupillary response to perisaccadic flashes (computed as in Fig. 3B). C: Spearman rank correlation between luminance of the flash and the seen/unseen report or the amplitude of the pupillary response (significant for most subjects; *P < 0.05), and partial correlation between the seen/unseen report and the pupillary response after controlling for the effect of luminance [nonsignificant (ns) with P > 0.05 in all but 1 subject]. Bars on right display the number of trials considered for these correlations. Error bars report SE of the correlation coefficient, computed as SE = √[(1 − r2)/(n − 2)].
Statistical analyses relied mainly on a linear-mixed model approach, motivated by the considerable sample size variability across subjects. In this approach, individual trials from all subjects are compared with a model comprising both the effect of experimental variables (“fixed effects”) and the variability across participants (“random effects”). The main fixed effects we analyzed are the categorical variable “delay” of flash relative to the saccade, which takes four values: no flash and pre-, peri-, or postsaccadic flash; a continuous variable “luminance” coding the luminance of the flash; and a dichotomous variable “perceptual report” indicating whether the subject had indicated having seen/not seen a flash on each trial. Random effects were coded by allowing subject-by-subject variations of both the slope and intercept for each of the fixed effects; we also used random effects to represent further variables that were not manipulated as in a full factorial design. For example, our first analysis compared saccade parameters and pupillary constrictions across all levels of the factor “delay” and we modeled the effect of luminance as a random effect (given that luminance in the no-flash condition was necessarily distinct from all flash luminance levels in the other conditions). We used standard MATLAB functions provided with the Statistics and Machine Learning Toolbox (R2015b, The MathWorks). Specifically, the function “fitlme(data, model)” fit the linear-mixed model to the data, yielding an object “lme” with associated method “anova” that returns F statistics and P values for each of the fixed effect terms.
RESULTS
While subjects made large saccades, we showed a flash of variable luminance and variable delays from the saccade onset. Figure 1, C–E, show that the flash had a minor influence on saccade parameters relative to catch trials where no flash was presented; as expected (e.g., Reingold and Stampe 2002), a presaccadic flash could interfere with saccade execution, leading to nonsignificantly delayed and larger saccades with significantly lower peak velocity [fixed effect “condition” with luminance and subject as random effects and contrasts evaluating the difference between presaccadic flashes and no flash: F(1,4088) = 8.786, P < 0.01]. However, saccade parameters in the other conditions (peri- and postsaccadic flashes) were closely matched to the no-flash condition (all P > 0.08).
We compared pupillary responses across conditions, and average traces of pupil diameter over time from saccade onset are shown in Fig. 2. The top trace in Fig. 2A shows the pupil modulation accompanying saccade execution with no flash presented. This consists of a progressive dilation leading up to saccade onset, probably associated with saccade preparation, followed by a marked constriction, similar to a light-evoked response and with unknown cause; in addition, a systematic disturbance is produced during the saccade, and it matches a known artifact of video-based eye-tracking systems. The other traces in Fig. 2A show pupil modulations recorded in trials when a flash did occur, so that the saccade-related modulation was combined with a light-evoked pupil response. Figure 2A shows traces averaged after subtracting the baseline pupil size for each trial (mean pupil size in the first 500 ms), whereas Fig. 2B shows the result of subtracting, from each trial, the average pupil trace in the no-flash condition. These correspond to two extreme hypotheses for describing the combination of the saccade-related and the light-evoked pupil modulation: 1) extreme subadditivity, where pupil size reflects only the largest component, or 2) perfect additivity, where the two components add up linearly and the pupil response to light is obtained by subtracting out the saccade-related modulation [as done in previous work (Lorber et al. 1965); see methods for the rationale behind the 2 analysis approaches].
Visual inspection of Fig. 2 indicates that, under either assumption, pupillary responses to perisaccadic flashes are smaller than for pre- and postsaccadic flashes (red traces are always less modulated than blue and green traces). The same conclusion is supported by the quantitative comparison of peak pupil constrictions, computed from traces in Fig. 2, A and B, and shown in Fig. 3, A and B, respectively. The delay of the flash relative to the saccade onset reliably affected pupillary constrictions (fixed effect “condition” with luminance and subject as random effects and contrasts evaluating the difference between peri- and pre/postsaccadic flashes); perisaccadic flashes evoked smaller responses compared with postsaccadic flashes [F(1,4088) = 36.397, P < 0.001 for Fig. 3A; F(1,4088) = 39.119, P < 0.001 for Fig. 3B] and compared with presaccadic flashes [F(1,4088) = 7.783, P < 0.01 for Fig. 3A; F(1,4088) = 39.513, P < 0.001 for Fig. 3B]. Similar effects in Fig. 3, A and B, imply that pupillary responses are suppressed perisaccadically, no matter whether we assume extreme subadditivity or perfect additivity between the different components of pupillary constrictions. The contrast of pre- vs. postsaccadic responses is significant, too [F(1,4088) = 5.336, P < 0.05 for Fig. 3A; F(1,4088) = 11.736, P < 0.001 for Fig. 3B]. Figure 2 indicates that the constriction in response to presaccadic flashes peaks just when there is the maximum saccade-related dilation, whereas for peri- and postsaccadic flashes the light response co-occurs with the saccade-related constriction. If the saccade-related modulation is not factored out, the response to presaccadic flashes is bound to be strongly reduced compared with postsaccadic flashes, as seen in Fig. 3A. However, when we do subtract out the saccade-related modulation, the resulting pupillary responses become larger presaccadically than postsaccadically (Fig. 3B). One possibility is that this subtraction leads to overcorrecting the saccade-related dilation, which could be smaller in the presaccadic flash than in the no-flash condition. This would be consistent with the saccade metrics results (Fig. 1, C–E), which suggests that the presaccadic flash interfered with saccade preparation and might therefore have impaired the associated pupil dilation (Wang et al. 2015).
A second analysis focused on data where a flash did occur and studied the effect of flash luminance on pupillary responses. This confirmed a significant effect of condition [pre- vs. peri- vs. postsaccadic flashes, F(2,3411) = 6.583, P < 0.01 for Fig. 3A; F(2,3411) = 8.335, P < 0.001 for Fig. 3B] and showed the expected effect of flash luminance [F(1,3411) = 44.583, P < 0.001 for Fig. 3A; F(1,3411) = 38.795, P < 0.001 for Fig. 3B], with no interaction between the two factors (P > 0.6 in both cases). This suggests that saccadic suppression of pupillary responses is a subtractive effect, in contrast with the divisive effect typically found for saccadic suppression of perceptual thresholds (Burr et al. 1994; Knoll et al. 2011; Watson and Krekelberg 2011)—but note that the luminance range tested here is small, and evidence for either model is weak.
The same analysis was applied to the other response we collected, the subjective visibility of the flashes (Fig. 3C, showing average proportions of “seen” responses as function of flash luminance and separately for each condition). While pre- and postsaccadic flashes were almost never missed (across all subjects, there were only 6 misses in 1,932 trials), perisaccadic flashes were often missed, in a proportion that varied with luminance (note that in catch trials with no flash presentation, false alarms were extremely infrequent: 2 in 675 trials). This resulted in a significant condition × luminance interaction [F(2,3411) = 288.654, P < 0.001].
Figure 4A shows the results from individual subjects, showing the difference of detection rate and the difference of pupillary responses (mm) and comparing perisaccadic and postsaccadic flashes (Fig. 4A, left) or perisaccadic and presaccadic flashes (Fig. 4A, right). While there is considerable variability across subjects, the suppression of pupillary responses is statistically significant in all but one case (significance evaluated by performing 2-sample t-tests and comparing, for each subject, single trial responses to peri- and pre/postsaccadic flashes; the number of trials in the perisaccadic flash condition is shown in Fig. 5C, right, with the same color coding and order of subjects).
Next we focused on trials with perisaccadic flash presentations. Despite the small variability of flash timing (in 95% of perisaccadic trials, the flash occurred between 6 and 18 ms from saccade onset), its exact delay from the saccade had a significant impact upon subjective reports of flash visibility [significant interaction of fixed effects delay and luminance, with subjects as random effects: F(1,1481) = 9.063, P < 0.01]. Figure 4B describes this interaction, plotting detection rate against the exact time of perisaccadic flashes from the saccadic onset, separately for flashes of different luminance: there is a negative trend, more pronounced for low-luminance flashes. The negative trend implies that the peak of suppression does not coincide with saccade onset but rather occurs >20 ms into the saccade. This is at odds with the time course of suppression typically found for detection of contrast patterns, peaking just before or at saccade onset (e.g., Diamond et al. 2000). However, such delayed suppression is consistent with the results of previous studies measuring detection of luminance flashes, where peak suppression clearly is delayed and occurs some 20–40 ms into the saccade (Lorber et al. 1965; Osaka 1987; Volkmann 1986).
In contrast with this effect on detection rate, the variation of pupillary responses with time (Fig. 4C) is less evident; coherently, the mixed-model analysis reveals no main effect of delay and no interaction between delay and luminance (both P > 0.5), only a main effect of luminance [F(1,1481) = 16.103, P < 0.001]. These results are consistent with different time courses of suppression for detection judgments and pupillary responses: faster for detection, implying strong variation of detection rates over a short time window (5–20 ms into the saccade, as measured here), and slower for pupillary responses. This is in line with Lorber et al.'s (1965) observation that the time course of saccadic suppression is different for perceptual and pupillary responses (tighter for the former), suggesting that saccadic suppression of perceptual and pupillary responses may be dissociable at the individual trial level.
To directly explore this possibility, we started by comparing pupillary responses to perisaccadic flashes that were reported as seen or unseen (Fig. 3D). Once the obvious effect of luminance is taken into account (i.e., in a mixed model with perceptual report as fixed effect and luminance, flash timing, and subject as random effects), there is no reliable difference between pupillary responses to perisaccadic seen and unseen flashes [F(1,1257) = 3.789, P > 0.05]. However, Fig. 5A shows that visibility thresholds varied considerably across participants (although all subjects were close to 100% correct in the no-flash and pre/postsaccadic flash conditions). We therefore narrowed trial selection further to look at luminance levels that, for each subject, led to an approximately equal number of trials with seen and unseen flashes; also in this case, we failed to find a statistically significant effect [F(1,301) = 1.768, P > 0.05].
This negative finding is not, of course, sufficient to conclude that pupillary and perceptual responses to perisaccadic flashes are independent. The ability to test this hypothesis depends on the specific model used to describe the relationship between the two responses, and at least some extreme possibilities can be excluded on the basis of our data.
First, we can rule out a model imposing the strongest possible relationship between perceptual reports and pupillary responses: where pupillary responses are intact vs. suppressed (in an all-or-none fashion) depending on the presence vs. absence of perceptual awareness. Besides predicting a difference between pupillary responses to seen and unseen perisaccadic flashes (which we failed to measure, see above), this model also predicts that pupillary responses should be unaffected by suppression whenever flashes are reported as seen. This is clearly not the case [fixed effect of “condition” contrasting seen perisaccadic vs. seen postsaccadic flashes, with luminance and subject as random effects: F(1,2354) = 35.781, P < 0.001].
Second, we can exclude a weaker model that releases the assumption of a direct mapping between the suppression of pupillary responses and presence/absence of perceptual awareness and simply assumes that pupillary responses should be absent when perceptual awareness is absent. We have strong evidence against this, too: even in the subset of trials where the perisaccadic flash is reported as unseen, pupillary responses are clearly detectable [fixed effect of “condition” contrasting unseen perisaccadic flashes vs. no-flash trials, with luminance and subject as random effects F(1,1257) = 3.960, P < 0.05] and sensitive to flash luminance [effect of luminance on unseen perisaccadic flashes with subject as random effect: F(1,582) = 6.376, P < 0.05].
Thus, whether seen or unseen, perisaccadic flashes lead to attenuated but still detectable pupillary responses; in other words, our data are only compatible with models in which the relationship between perceptual and pupillary responses has an unspecified (and small) effect size. We estimated this effect size by studying the correlation of pupillary and perceptual responses at the individual subject level. Figure 5 shows that both responses varied with flash luminance (as shown for the data pooled across subjects discussed above) and were indeed well correlated with luminance (on average [95% confidence interval] R = 0.41 [0.24, 0.58] and 0.26 [0.17, 0.36], respectively, close to the 0.3 value defined as a “medium”-sized effect in Cohen's classification; Cohen 1988). When this effect of luminance is controlled for, however, the remaining partial correlation between pupillary responses and perceptual reports becomes very small (on average R = 0.07 [95% CI −0.004, 0.14]), nonsignificantly different from 0 [1-sample t-test, t(13) = 1.92, P = 0.08] and close to the 0.1 value termed “small” in Cohen's classification (Cohen 1988). In conclusion, even if there is no practical way of completely excluding a relationship between the suppression of perceptual and pupillary responses, our results indicate that if such a relationship exists it is a trivially small one.
DISCUSSION
We flashed lights during or before/after a saccade while monitoring pupil diameter. In agreement with previous observations (Lorber et al. 1965; Zuber et al. 1966), we find that the pupillary constrictions in response to light flashes are strongly suppressed during saccades. In control trials where no flash was presented, we find that the mere execution of a saccade is sufficient to generate a pupillary modulation—noted and described previously (Lorber et al. 1965; Mathôt et al. 2015; Zuber et al. 1966). This implies that responses to flashes presented at saccade onset reflect the combination of two pupil responses, related to light and to the saccade. Previous work assumed that the combination was linear and factored out the second by subtracting, from the raw traces, the pupil modulation observed in saccade-only trials (Lorber et al. 1965; Zuber et al. 1966). We show that releasing this linearity assumption does not change the conclusion: pupillary constrictions evoked by perisaccadic flashes are suppressed relative to pre- and postsaccadic flashes, even if we fail to discount the effect of the saccade-related modulation. Our Fig. 2 also confirms that subtracting the latter from the raw pupil traces has the advantage of reducing the complexity of waveforms, yielding traces that match the typical light response well (Bitsios et al. 1996). This allows for summarizing pupillary responses with established indexes like the peak pupil constriction, which we use for all our analyses. These show that the suppression is approximately constant across the tested luminance range (∼50–100 cd/m2, typical of everyday computer use and TV watching): a reduction of ∼0.2 mm, which represents up to 90% of the pupillary responses evoked by the test flashes.
Because we simultaneously monitored the subjective flash visibility (perceptual detection rate), our measurements offer an opportunity to test the relationship between the saccadic suppression of perceptual and pupillary responses. We find that such a relation is not tight. Our data are consistent with Lorber et al.'s (1965) report that the temporal dynamics of saccadic suppression of pupillary responses is different from that of perceptual responses. In addition, we find that pupillary responses to perisaccadic flashes do not differ depending on the perceptual report—whether the flash was seen or not seen. Pupillary responses remain clearly detectable and show the expected luminance dependence even when flashes are suppressed from perceptual awareness. We cannot, of course, rule out all possible models that impose any arbitrarily small relationship between pupillary and perceptual responses. However, we can look at their trial-by-trial correlation to estimate the effect size of such a relationship. Once we factor out the effect of luminance, with which both perceptual and pupillary responses are expected to correlate, the residual correlation between the two responses is only ∼0.1—if at all present, the relationship is a small one, corresponding to <2% explained variance (Cohen 1988).
A dissociation between the saccadic suppression of conscious vision and other forms of visual responses was previously proposed in Watson and Krekelberg's study (2009), where the suppression of a line stimulus from conscious perception did not eliminate its “shape contrast” effect or the ability to bias the apparent shape of a subsequently presented ellipse. Our results reinforce the evidence that the content of our consciousness is not the only representation available in the visual system; distinct representations appear to be accessible to support nonconscious responses (Goodale and Milner 1992; Mishkin et al. 1983). Pupillary responses are an extreme example of these—even if there is evidence that they are not reflexes (Binda and Murray 2015a), they are still completely automatic responses that escape voluntary control (Loewenfeld 1993). Another example is open-loop pointing, which a previous study showed to differentiate from conscious perception of perisaccadic stimuli (Burr et al. 2001). In this case, subjects reported the perceived location—rather than the visibility—of perisaccadic stimuli; there were strong localization biases for both subjective reports and pointing responses, but the two were systematically different.
By suggesting that saccades differentially affect conscious and nonconscious visual processing, these observations may seem incompatible with the hypothesis that saccades affect visual processing by acting very early—even before the visual signal reaches the cortex (Burr et al. 1994; Wurtz 2008). However, the discrepancy may be resolved by assuming that visual pathways supporting conscious vision vs. other forms of processing diverge even earlier—subcortically, with unconscious responses relying on an extrageniculate pathway possibly involving the superior colliculus (Tamietto et al. 2010).
Given that there is no consensus either on the site of saccadic suppression or on the divergence between pathways supporting conscious vs. unconscious visual functions, the neural mechanisms underlying the suppression of pupillary responses we observe here remain unclear. We nevertheless note that some relatively subtle features of our results fit with the hypothesis that saccadic suppression primarily targets a cortically mediated component of the pupillary light response. The pupillary response we studied is a transient constriction, and this is likely a combination of a light-dependent constriction (which would have been sustained had our stimulus been a constant light increment rather than a brief flash) with a non-luminance-dependent transient constriction. The latter can be evoked by stimuli such as changes of chromaticity or motion direction and, primarily, by gratings (Barbur et al. 1992; Sahraie and Barbur 1997). The “grating” response has a low-pass behavior (Young and Kennish 1993), meaning that it is most responsive to low spatial frequencies (the lowest being a full-screen stimulus like a luminance flash); it is quickly saturated with contrast (Young and Kennish 1993), and its maximal amplitude is usually 0.1–0.2 mm. Thus assuming that this component is selectively suppressed during saccades would be consistent with an effect of suppression of ∼0.2 mm approximately constant across luminance levels, just as we observed here—but note that the limited range of tested luminance levels does not allow for excluding alternative models of the suppression effect, e.g., a divisive effect as seen in psychophysics (e.g., Knoll et al. 2011). It is interesting to note that this grating response has been associated with the magnocellular pathway (Young and Kennish 1993), which is believed to be the main target of saccadic suppression (Burr et al. 1994). Also, the grating response is strongly attenuated in patients with lesions of the visual cortex—indicative of a cortical source—and, in some of these patients, a dissociation was found between grating responses and conscious vision—the amplitude of the pupillary response correlating with unconscious visual discrimination abilities or “blindsight” (Sahraie et al. 2013). If this component were suppressed during saccades, then one would not necessarily expect a correlation with the suppression of conscious vision. Whether pupillary suppression correlates better with unconscious visual processing, such as revealed by the Watson and Krekelberg study (2009), remains an open question.
Here we focused on pupillary responses to the flash stimuli and have shown that the mere presence of pupil modulation related to saccade execution cannot in itself influence our estimates of saccadic suppression. However, further studies are necessary to investigate this eye movement-related pupil modulation, especially since its cause and function are at present unknown. It cannot be entirely explained either by 1) the eye-position artifact (Hayes and Petrov 2015), evident as a rapid, small pupil change during the saccade, or 2) the effort of preparing the saccade execution (Wang and Munoz 2015), which consists of a progressive dilation preceding the saccade. Neither of these effects explains the prolonged constriction after the eye has reached its final postsaccadic position. Because pupil constriction is known to accompany near focus (Loewenfeld 1993; Marg and Morgan 1949), Zuber et al. (1966) suggested that this modulation reflects a change of focal plane during a saccade. More recently, Mathôt et al. (2015) advanced the hypothesis that the constriction reflects a “grating” response instead, elicited by the spurious motion of retinal images produced by the eye movement. Available evidence is insufficient to support any of these proposals. It is also interesting to note that a similar constriction also accompanies eyeblinks (e.g., Hupe et al. 2009), which are associated with perceptual suppression like saccades, suggesting that understanding the nature of this pupil modulation might ultimately be relevant to explaining the suppression of light responses.
In conclusion, the pupillary response to light flashes shows a robust suppression during saccades, with features that deviate in interesting ways from the suppression of conscious vision. This highlights the complexity of pupillary responses, which integrate diverse sources of information. It also provides further support to the idea that saccades may produce different effects on visual pathways supporting conscious perception and those supporting other visual functions, e.g., (oculo)motor responses.
GRANTS
The research leading to these results has received funding from the Italian Ministry of University and Research under the project “Futuro in Ricerca,” grant agreement no. RBFR1332DJ, from the project ECSPLAIN—European Research Council under the Seventh Framework Programme (FPT/2007–2013), grant agreement no. 338866, from the Fondazione Roma, and from the Tuscany Region Pegaso Scholarship 2013.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.B. and P.B. performed experiments; A.B. and P.B. analyzed data; A.B. and P.B. interpreted results of experiments; A.B. and P.B. edited and revised manuscript; A.B. and P.B. approved final version of manuscript; P.B. conception and design of research; P.B. prepared figures; P.B. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Profs. M. C. Morrone and D. C. Burr for their help in all aspects of the research and for providing laboratory tools and spaces.
REFERENCES
- Barany EH, Hallden U. Phasic inhibition of the light reflex of the pupil during retinal rivalry. J Neurophysiol 11: 25–30, 1948. [DOI] [PubMed] [Google Scholar]
- Barbur J. Learning from the pupil: studies of basic mechanisms and clinical applications. In: The Visual Neurosciences, edited by Chalupa L, Werner J. Cambridge, MA: MIT Press, 2004, vol. 1, p. 641–656. [Google Scholar]
- Barbur JL, Harlow AJ, Sahraie A. Pupillary responses to stimulus structure, color and movement. Ophthalmic Physiol Opt 12: 137–141, 1992. [DOI] [PubMed] [Google Scholar]
- Binda P, Murray SO. Keeping a large-pupilled eye on high-level visual processing. Trends Cogn Sci 19: 1–3, 2015a. [DOI] [PubMed] [Google Scholar]
- Binda P, Murray SO. Spatial attention increases the pupillary response to light changes. J Vis 15: 1, 2015b. [DOI] [PubMed] [Google Scholar]
- Binda P, Pereverzeva M, Murray SO. Attention to bright surfaces enhances the pupillary light reflex. J Neurosci 33: 2199–2204, 2013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda P, Pereverzeva M, Murray SO. Pupil constrictions to photographs of the sun. J Vis 13: 8, 2013b. [DOI] [PubMed] [Google Scholar]
- Binda P, Pereverzeva M, Murray SO. Pupil size reflects the focus of feature-based attention. J Neurophysiol 112: 3046–3052, 2014. [DOI] [PubMed] [Google Scholar]
- Bitsios P, Prettyman R, Szabadi E. Changes in autonomic function with age: a study of pupillary kinetics in healthy young and old people. Age Ageing 25: 432–438, 1996. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997. [PubMed] [Google Scholar]
- Burr DC, Morrone MC, Ross J. Selective suppression of the magnocellular visual pathway during saccadic eye movements. Nature 371: 511–513, 1994. [DOI] [PubMed] [Google Scholar]
- Burr DC, Morrone MC, Ross J. Separate visual representations for perception and action revealed by saccadic eye movements. Curr Biol 11: 798–802, 2001. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Erlbaum, 1988. [Google Scholar]
- Cornelissen FW, Peters EM, Palmer J. The Eyelink Toolbox: eye tracking with MATLAB and the Psychophysics Toolbox. Behav Res Methods Instrum Comput 34: 613–617, 2002. [DOI] [PubMed] [Google Scholar]
- Diamond MR, Ross J, Morrone MC. Extraretinal control of saccadic suppression. J Neurosci 20: 3449–3455, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamlin PD, Clarke RJ. The pupillary light reflex pathway of the primate. J Am Optom Assoc 66: 415–418, 1995. [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci 15: 20–25, 1992. [DOI] [PubMed] [Google Scholar]
- Hayes TR, Petrov AA. Mapping and correcting the influence of gaze position on pupil size measurements. Behav Res Methods (May 8, 2015). doi: 10.3758/s13428-015-0588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupe JM, Lamirel C, Lorenceau J. Pupil dynamics during bistable motion perception. J Vis 9: 10, 2009. [DOI] [PubMed] [Google Scholar]
- Knoll J, Binda P, Morrone MC, Bremmer F. Spatiotemporal profile of peri-saccadic contrast sensitivity. J Vis 11: 15, 2011. [DOI] [PubMed] [Google Scholar]
- Laeng B, Endestad T. Bright illusions reduce the eye's pupil. Proc Natl Acad Sci USA 109: 2162–2167, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeng B, Sulutvedt U. The eye pupil adjusts to imaginary light. Psychol Sci 25: 188–197, 2014. [DOI] [PubMed] [Google Scholar]
- Loewenfeld I. The Pupil: Anatomy, Physiology, and Clinical Applications. Detroit, MI: Wayne State Univ. Press, 1993. [Google Scholar]
- Lorber M, Zuber BL, Stark L. Suppression of pupillary light reflex in binocular rivalry and saccadic suppression. Nature 208: 558–560, 1965. [Google Scholar]
- Marg E, Morgan MW Jr. The pupillary near reflex; the relation of pupillary diameter to accommodation and the various components of convergence. Am J Optom Arch Am Acad Optom 26: 183–198, 1949. [PubMed] [Google Scholar]
- Mathôt S, Dalmaijer E, Grainger J, Van der Stigchel S. The pupillary light response reflects exogenous attention and inhibition of return. J Vis 14: 7, 2014. [DOI] [PubMed] [Google Scholar]
- Mathôt S, Melmi JB, Castet E. Intrasaccadic perception triggers pupillary constriction. PeerJ 3: e1150, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathôt S, van der Linden L, Grainger J, Vitu F. The pupillary light response reveals the focus of covert visual attention. PLoS One 8: e78168, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG, Macko KA. Object vision and spatial vision: two cortical pathways. Trends Neurosci 6: 414–417, 1983. [Google Scholar]
- Naber M, Alvarez GA, Nakayama K. Tracking the allocation of attention using human pupillary oscillations. Front Psychol 4: 919, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naber M, Nakayama K. Pupil responses to high-level image content. J Vis 13: 7, 2013. [DOI] [PubMed] [Google Scholar]
- Osaka N. Variation of saccadic suppression with target eccentricity. Ophthalmic Physiol Opt 7: 499–501, 1987. [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997. [PubMed] [Google Scholar]
- Reingold EM, Stampe DM. Saccadic inhibition in voluntary and reflexive saccades. J Cogn Neurosci 14: 371–388, 2002. [DOI] [PubMed] [Google Scholar]
- Richards W. Attenuation of the pupil response during binocular rivalry. Vision Res 6: 239–240, 1966. [DOI] [PubMed] [Google Scholar]
- Ross J, Morrone MC, Goldberg ME, Burr DC. Changes in visual perception at the time of saccades. Trends Neurosci 24: 113–121, 2001. [DOI] [PubMed] [Google Scholar]
- Sahraie A, Barbur JL. Pupil response triggered by the onset of coherent motion. Graefes Arch Clin Exp Ophthalmol 235: 494–500, 1997. [DOI] [PubMed] [Google Scholar]
- Sahraie A, Trevethan CT, MacLeod MJ, Urquhart J, Weiskrantz L. Pupil response as a predictor of blindsight in hemianopia. Proc Natl Acad Sci USA 110: 18333–18338, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamietto M, Cauda F, Corazzini LL, Savazzi S, Marzi CA, Goebel R, Weiskrantz L, de Gelder B. Collicular vision guides nonconscious behavior. J Cogn Neurosci 22: 888–902, 2010. [DOI] [PubMed] [Google Scholar]
- Volkmann FC. Human visual suppression. Vision Res 26: 1401–1416, 1986. [DOI] [PubMed] [Google Scholar]
- Wang CA, Brien DC, Munoz DP. Pupil size reveals preparatory processes in the generation of pro-saccades and anti-saccades. Eur J Neurosci 41: 1102–1110, 2015. [DOI] [PubMed] [Google Scholar]
- Wang CA, Munoz DP. A circuit for pupil orienting responses: implications for cognitive modulation of pupil size. Curr Opin Neurobiol 33: 134–140, 2015. [DOI] [PubMed] [Google Scholar]
- Watson T, Krekelberg B. An equivalent noise investigation of saccadic suppression. J Neurosci 31: 6535–6541, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson TL, Krekelberg B. The relationship between saccadic suppression and perceptual stability. Curr Biol 19: 1040–1043, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz RH. Neuronal mechanisms of visual stability. Vision Res 48: 2070–2089, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz RH, Joiner WM, Berman RA. Neuronal mechanisms for visual stability: progress and problems. Philos Trans R Soc Lond B Biol Sci 366: 492–503, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RS, Kennish J. Transient and sustained components of the pupil response evoked by achromatic spatial patterns. Vision Res 33: 2239–2252, 1993. [DOI] [PubMed] [Google Scholar]
- Zuber BL, Stark L, Lorber M. Saccadic suppression of the pupillary light reflex. Exp Neurol 14: 351–370, 1966. [DOI] [PubMed] [Google Scholar]