Abstract
Functional magnetic resonance imaging (fMRI) is a noninvasive tool used to probe cognitive and affective processes. Although fMRI provides indirect measures of neural activity, the advent of fMRI has allowed for 1) the corroboration of significant animal findings in the human brain, and 2) the expansion of models to include more common human attributes that inform behavior. In this review, we briefly consider the neural basis of the blood oxygenation level dependent signal to set up a discussion of how fMRI studies have applied it in examining cognitive models in humans and the promise of using fMRI to advance such models. Specifically, we illustrate the contribution that fMRI has made to the study of reward processing, focusing on the role of the striatum in encoding reward-related learning signals that drive anticipatory and consummatory behaviors. For instance, we discuss how fMRI can be used to link neural signals (e.g., striatal responses to rewards) to individual differences in behavior and traits. While this functional segregation approach has been constructive to our understanding of reward-related functions, many fMRI studies have also benefitted from a functional integration approach that takes into account how interconnected regions (e.g., corticostriatal circuits) contribute to reward processing. We contend that future work using fMRI will profit from using a multimodal approach, such as combining fMRI with noninvasive brain stimulation tools (e.g., transcranial electrical stimulation), that can identify causal mechanisms underlying reward processing. Consequently, advancements in implementing fMRI will promise new translational opportunities to inform our understanding of psychopathologies.
Keywords: striatum, nucleus accumbens, reinforcement, corticostriatal projections, brain stimulation
functional magnetic resonance imaging (fMRI) is an excellent tool to probe neural function. Even though it indirectly measures neural activity by tracking correlative hemodynamic changes, it has the capability to map task-dependent whole-brain activation. This feature, along with its noninvasive nature, makes fMRI a tremendous asset to the study of cognitive and affective processes in both healthy and patient human populations. Indeed, the application of fMRI to study the human brain has allowed for 1) confirmation of core findings from nonhuman animal studies that have shaped models of cognitive and affective processing; and 2) extension of those findings and new directions for such models by probing characteristics more accessible in humans. One noteworthy example is the study of reward processing, which has been informed by a rich nonhuman animal literature employing an array of techniques, from selective lesions to electrophysiological recordings, to delineate a neural reward circuit (e.g., Berridge and Robinson 2003; Robbins and Everitt 1996; Schultz 2006). An explosion of fMRI studies over the last decade or so (Fig. 1) has substantiated this reward circuit in the human brain, with emphasis on higher-level functions that are more commonly observed in humans. Many of these fMRI studies have also examined deficits in the reward circuit in patient populations. As a result, the use of fMRI to study reward processing has greatly expanded our understanding of its neural basis in humans.
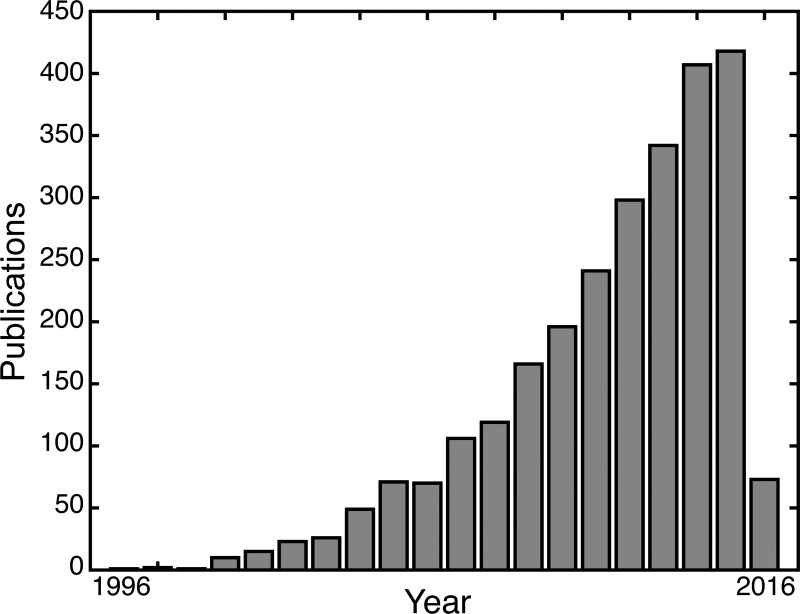
Fig. 1.
Proliferation of functional magnetic resonance imaging (fMRI) studies in reward processing. The use of fMRI to study reward processing has been increasingly popular over the past 20 yr. During this time, the number of publications on fMRI and reward has increased quasi-exponentially. We note that the shown data were extracted from http://www.ncbi.nlm.nih.gov/pubmed/ on February 13, 2016 using the search term “(fMRI OR functional magnetic resonance imaging) AND reward.”
Despite the many advantages that fMRI has afforded the study of reward processing, there are some inherent challenges that discount the full promise of fMRI. For instance, the neurophysiological nature of the fMRI signal can cloud its potential neural interpretations. While we address these limitations, our synthesis of the literature highlights the promise of fMRI in advancing models of cognitive and affective processes. First, we describe the fMRI blood oxygenation level dependent (BOLD) signal and consider potential pitfalls related to its neural interpretations. Second, we illustrate the use of fMRI in both confirming key findings and extending such findings to advance models of cognitive and affective processing. We specifically anchor our discussion on the study of reward processing as an exemplar topic because it has garnered considerable experimental efforts across techniques and species. In the last section, we highlight the promise of fMRI in studying reward processing and describe how it fits into the progressive multimodal and across-technique approach to study such psychological phenomena.
A Neural Interpretation of the BOLD Signal in fMRI
fMRI detects neural activation by measuring changes in the BOLD signal. The BOLD signal is coupled to hemodynamic changes such as blood flow (Logothetis and Wandell 2004) and decreasing levels of deoxygenated hemoglobin (deoxyhemoglobin; Ogawa et al. 1992), whose paramagnetic nature allows BOLD to indirectly track the underlying neural activity. Taking into account the many comprehensive and informative reviews (Buxton et al. 2004; Logothetis 2008; Logothetis and Wandell 2004) on the technical underpinnings of fMRI and its BOLD signal, we provide below a succinct and generalized account of the neural interpretation of the BOLD signal and how it is typically analyzed in fMRI experiments to infer neural functions. In doing so, we hope to provide readers of all backgrounds with sufficient understanding of the fMRI findings we present throughout the review and appreciation for the advantages of fMRI we subsequently discuss for the rest of the paper.
The cellular underpinnings of the BOLD signal.
Throughout various fMRI studies, most experimental protocols observe BOLD signals that correspond to localized increases in cerebral blood flow (CBF). These CBF increases, coupled with smaller positive changes in the cerebral metabolic rate of oxygen consumption, lead to the production of a hemodynamic response (Buxton and Frank 1997; Hoge et al. 1999; Raichle et al. 1976). Importantly, unlike the immediate nature of neuronal spiking activity (Lauritzen and Gold 2003), the hemodynamic response has a lagged response that begins approximately 2 s after neural stimulation and peaks 4–6 s thereafter (Bandettini et al. 1992). Although the hemodynamic response was initially interpreted to represent neuronal output (Rees et al. 2000), subsequent studies soon reported robust BOLD signals in the absence of spiking activity (Rauch et al. 2008; Viswanathan and Freeman 2007), fueling the interpretation of the BOLD signal as an indicator of the underlying local field potential (LFP; Goense and Logothetis 2008; Logothetis 2002, 2008; Magri et al. 2012; Nir et al. 2007). Because LFP underlines the synaptic inputs and dendritic processing in a particular region (Berens et al. 2010), the LFP-driven hemodynamic response is more strongly encoded by aggregate cellular activity within a localized excitation-inhibition network rather than single-cell activity (see review by Logothetis and Panzeri 2015). In short, the neurophysiological underpinnings of the BOLD signal are thought to reflect LFP during neural stimulation.
Some experimental protocols also detect negative BOLD responses (NBR) that are postulated to reflect neuronal suppression (Wade 2002). Although there is no widely accepted neurophysiological explanation of NBR, it is clear that one cannot simply assume that NBR is the neurophysiological inverse of a hemodynamic response (Mullinger et al. 2014). The current neuronal explanation of NBR is divided into two camps of thought. On one end of the debate, Shmuel and colleagues (2006) observed that NBR was tightly coupled to local decreases in LFP, which led to the view that NBR is a representation of neural deactivation (Hayden et al. 2009; Klingner et al. 2010; Mckiernan et al. 2003; Pasley et al. 2007). On the opposite end lie those who, guided by Logothetis (2008) and his metabolic-increasing excitation-inhibition microcircuit viewpoint, put forth the argument that NBR encodes underlying neural activation (Kim et al. 2014; Schridde et al. 2008; Shulman et al. 2007). In essence, the interaction between CBF and blood volume changes encodes the excitation-inhibition balance, giving rise to NBR (Huber et al. 2014). Despite the irresolute nature of this debate, continued progress on understanding the neural nature of NBR is important to provide more insights on the BOLD signal and to further refine the role that different neural regions play in cognitive processes.
From BOLD signals to inferences on brain function.
Neuroimaging studies tend to visualize the hemodynamic response (e.g., plot the time series of the data) but more commonly report parameter estimates summarizing the fit of a statistical model to the BOLD data (e.g., Fig. 2). To obtain these parameter estimates, the known stimulus functions based on preset experimental conditions are first convolved with a canonical hemodynamic response function to establish the predicted BOLD responses. Predicted BOLD responses are subsequently used to test, under the framework of the general linear model (Friston et al. 1994; Worsley and Friston 1995), whether activity in brain regions is related to any of the BOLD input functions (e.g., the raw data). Most fMRI studies report statistical fit of the BOLD functions as a series of parameter estimates, which can have both positive and negative deflections relative to prestimulus activation (for details on fMRI analysis and issues such as multiple comparisons, please see Poldrack et al. 2011). It is important to distinguish these negative deflections from NBR, because negative parameter estimates reflect relative deviations from the implicit baseline in the model rather than the measured BOLD signals. In addition, we note that statistical inference in fMRI studies can suffer from many of the same problems that affect neurophysiological studies, including circular analyses (Kriegeskorte et al. 2009) and erroneous interactions (Nieuwenhuis et al. 2011). Nonetheless, these parameter estimates derived from BOLD responses have served fMRI researchers well, as we will discuss in detail throughout the rest of this review, in advancing the understanding of neural activity during task-induced cognitive and affective processes.
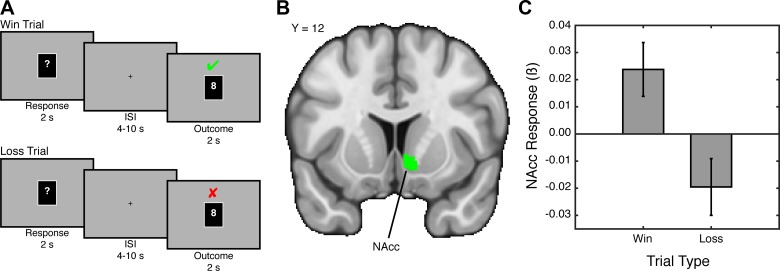
Fig. 2.
Gains and losses modulate activation in the striatum. A: a popular approach to studying reward processing employs a card-guessing task. In this paradigm, subjects are presented with a card and asked to guess whether the number on the card (range: 1–9) will be higher or lower than 5. If the subject guesses correctly, he or she wins money. However, if the subject guesses incorrectly, he or she loses money. B: contrasting positive outcomes or win trials against negative outcomes or loss trials reveals activation within the striatum. Here we focus on the nucleus accumbens (NAcc). C: within the NAcc, the responses to wins (depicted with parameter estimates) are higher than the responses to losses. Figure used data from Fareri et al. (2012).
Using fMRI to Study Reward Processing in the Striatum
Given its noninvasive nature and potential to visualize function in the whole brain, fMRI became a powerful and practical tool to study cognitive and affective processes in humans. Over the years, the use of fMRI proved to be an important asset in studying such processes as it afforded a way to confirm basic findings characterized in nonhuman animal studies and extend such findings to appreciate various aspects of human life, from distinctively human stimuli (e.g., money) to behaviors (e.g., cognitive emotion regulation) that translated to better understanding of neuropsychiatric disorders (e.g., mood disorders). One such phenomenon that benefitted from the proliferation of fMRI studies is reward-related processing and its relation to decision making (Fig. 1). Rewards can be broadly defined as stimuli that elicit approach behaviors, induce subjective feelings of pleasure during consumption, and lead to reinforcement of cues and actions (Schultz 2006, 2015). The regulation of the psychological and behavioral responses to rewarding stimuli is coordinated by a collection of cortical and subcortical structures that together make up the brain's reward circuit (see review by Haber and Knutson 2010). At the core of such circuit is the striatum (Fig. 3), a subcortical structure that is involved in reward-related learning and how it informs approach and consummatory behaviors.
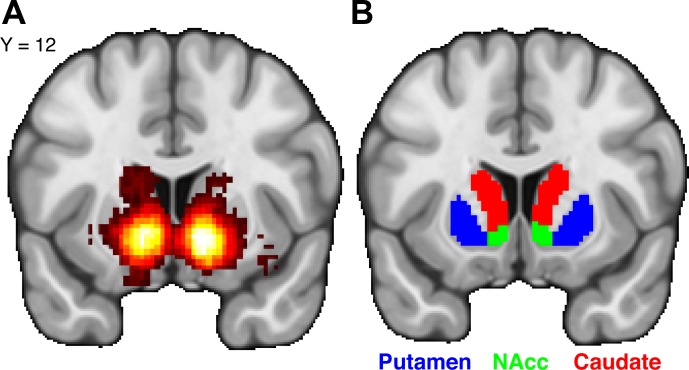
Fig. 3.
Reward processing and the striatum. A: a large-scale meta-analysis of 506 neuroimaging studies indicates a selective association between the term “reward” and striatal activation (Yarkoni et al. 2011). These observations help illustrate the reliability of neuroimaging evidence in demonstrating the involvement of the striatum in reward processing. B: anatomical subdivisions of the striatum in the human brain. These subdivisions include the putamen (blue), NAcc (green), and caudate (red).
Building on a large repertoire of studies using nonhuman animal models (Daw and Doya 2006; Hikosaka et al. 1989; Robbins and Everitt 1996; Schultz et al. 1997), many fMRI experimental efforts have focused on elucidating how the striatum, the input unit of the basal ganglia and a structure with strong connections with cortical regions and midbrain dopaminergic centers (Middleton and Strick 2000; Wang et al. 2015b), contributes to reward processing (Fig. 3; e.g., for review, see Bartra et al. 2013; Clithero and Rangel 2014; Delgado 2007; Haber and Knutson 2010; Smith and Delgado 2015). In the following section, we will provide a brief discussion of research findings from both nonhuman animals and humans, focusing on the striatal role in reward-related processing, particularly in approach and consummatory behavior, and how they are shaped by learning.
Approach behaviors in reward processing.
Approaching a potential reward is a typical behavior observed across species that is elicited by the anticipation of the pleasure a reward may bring. In nonhuman animals, this was initially characterized in studies where presentation of a conditioned stimulus that predicted a reward elicited a conditioned approach response in pigeons (Brown and Jenkins 1968; Williams and Williams 1969) and rats (Locurto et al. 1976; Peterson et al. 1972). This conditioned cue-induced approach behavior was found to depend on the integrity of the striatum, such that lesion (Parkinson et al. 1999) or dopaminergic depletion in the ventral parts of the striatum, particularly the nucleus accumbens (NAcc) (Parkinson et al. 2002), decreased approach behavior to a conditioned stimulus paired with reward (Di Ciano et al. 2001; Parkinson et al. 2000).
In nonhuman primates, a similar link between striatal neurophysiological signals and anticipatory responses to reward-related cues that can elicit approach behaviors have been observed. For instance, striatal neurons have been found to increase their firing rates during the anticipatory phase preceding reward delivery, highlighting a potential influence on reward-seeking approach behaviors (Ito and Doya 2015; Kawagoe et al. 1998; McGinty et al. 2013; Samejima et al. 2005). Interestingly, distinct subsections of the striatum can show different contributions to approach behaviors. Ventral striatal neurons, for example, have been shown to increase their firing rates in response to cues predicting rewards (Cromwell and Schultz 2003; Hassani et al. 2001; Hollerman et al. 1998; Schultz et al. 1992). In contrast, dorsal striatal neuronal responses have been linked with tracking the values of available actions for reward attainment (Lau and Glimcher 2007, 2008; Tai et al. 2012).
In humans, initial fMRI studies served to replicate findings from nonhuman animals, identifying the striatum as a key region involved in responding to cues that predicted potential rewards and could exert influences on approach behavior. For instance, initial efforts showed that BOLD responses in the ventral striatum, which includes the NAcc (Fig. 3), were correlated with craving of potential drug rewards (Breiter et al. 1997), relating to a motivational construct of “wanting”, which can lead to approach behaviors such as reward seeking (Berridge and Robinson 1998). This was quickly followed by reports of increased BOLD responses in the striatum to conditioned cues that predict potential primary rewards, including pleasant liquids (O'Doherty et al. 2002) or odors (Gottfried et al. 2002), and secondary rewards such as money (Knutson et al. 2001). As in nonhuman animals, distinct contributions of subsections of the striatum have also been reported, with the dorsal striatum, encompassing the caudate nucleus and putamen (Fig. 3), being more specifically recruited when participants performed an action (e.g., pressing a button) in response to cues predicting reward (O'Doherty et al. 2004; Tricomi et al. 2004). While dorsal striatum activity has been linked to the encoding of action values used in action selection during reward-seeking behaviors (FitzGerald et al. 2012), ventral striatum activity has been shown to correlate with participant's passive viewing responses to conditioned stimuli (Chumbley et al. 2014). This observation is in line with the actor-critic model (Sutton and Barto 1998), suggesting that the dorsal striatum can serve a potential function of an “actor” that facilitates action selection, whereas the ventral striatum can serve as a “critic” to guide future reward attainment (O'Doherty et al. 2004).
More recently, fMRI studies have extended these initial findings of striatal involvement in eliciting reward-related approach behavior to demonstrate how they relate to everyday human behaviors. Increased activation in the striatum to pictures of appetizing food items, for example, has been found to correlate with increased reward-seeking behavior (as assessed by greater weight gain months after initial data acquisition; Demos et al. 2012). Similarly, increased activation in the striatum to positive, arousing images (Knutson et al. 2008) and cues that predict monetary rewards (Kuhnen and Knutson 2005) are associated with elevated risk-taking behaviors. The relation between striatal BOLD activity and risk-taking behaviors is observed in different domains, such as drug-related cues elevating craving responses (Sinha et al. 2007), which can have an influence in maladaptive behaviors such as drug-seeking. Finally, this response in the striatum is also dependent on the state of an individual (e.g., stressed: Porcelli et al. 2012; or sleep deprived: Venkatraman et al. 2011) or context in which a reward is perceived. For example, the presence of a peer can change reward-related responses in the striatum (Chein et al. 2011; Fareri et al. 2012), which corresponds with increased risk-taking behaviors in some cases (e.g., adolescence; Chein et al. 2011). These studies collectively highlight the use of fMRI in understanding how the brain processes reward-related information and how it contributes to approach behaviors that complement and extend the knowledge gained from nonhuman animal studies.
Consummatory behaviors in reward processing.
A consummatory behavior occurs during the delivery or receipt of a reward. The consumption of rewards, such as food and sex, induces a pleasurable sensation which can be experimentally elicited in rats when neural regions such as the septal areas (Olds and Milner 1954) and NAcc (Olds 1956) are stimulated. Comparable studies in nonhuman primates (Porter et al. 1959) and humans (Bishop et al. 1963) have similarly shown that electrical stimulations delivered to the NAcc generates a pleasurable sensation. The hedonic aspects of reward are generally associated with opiate receptors in the NAcc (Peciña and Berridge 2000), but more general affective processing that can inform the reinforcement of actions is evident during reward consumption, being associated to dopamine release into the NAcc (Nakahara et al. 1989) and the firing of striatal neurons (Apicella et al. 1991; Hikosaka et al. 1989; Klein and Platt 2013; Schultz et al. 1993). In rodents, an interesting distinction is further noted where lesions to ventral parts of the striatum disrupt approach behaviors, whereas lesions to the more dorsal parts disrupt consummatory behaviors (Everitt and Robbins 2005).
In humans, reward consumption is typically probed during the outcome phase of a given task, where, for example, a participant may receive the resolution of a decision (e.g., monetary gain) or be presented with a stimulus that carries a positive value (e.g., liquids when thirsty, pleasant pictures). Several fMRI studies have observed activation in the striatum in response to a rewarding or positive outcome (Fig. 2). This extends to a variety of stimuli, from the most basic such as food (e.g., chocolate; McCabe et al. 2010), money (Fig. 3; Delgado et al. 2000), or just positive feedback (e.g., correct; Delgado et al. 2004; Foerde and Shohamy 2011) related to goal achievement (Tricomi and Fiez 2008) to the more abstract positive feelings elicited from observing a beautiful face (Smith et al. 2010), art (Lacey et al. 2011), receiving social feedback (Izuma et al. 2008), or even thinking about the self, such as when one discloses information about oneself to another (Tamir and Mitchell 2012), or recalls autobiographic positive memories (Speer et al. 2014). Interestingly, individual differences in the striatal BOLD signals associated with the consumption of such rewards have been shown to be very important in understanding questions of human behavior and health that can be studied with fMRI. For instance, striatal responses to evaluation of the self from others have been linked with pubertal status and age (Jankowski et al. 2014), while simple responses to monetary gains and losses in the striatum correlated positively with the sustainment of real-world positive emotions (Heller et al. 2015) and negatively with early life stress, such as emotional neglect (Hanson et al. 2015b). Taken together, these findings highlight the contribution of using fMRI to explore reward-related processing in the human brain and its links to behavior and health outcomes.
Reward-related learning.
The observations of the striatum responding to stimuli that predict rewarding outcomes support a prominent role for striatal circuits in reward-based learning. Indeed, the striatum has been implicated in a variety of learning studies involving cues that predict reward (e.g., O'Doherty et al. 2004) to probabilistic reinforcement learning tasks where feedback that allows for correction of behavior is presented, both in fMRI studies (e.g., Dickerson et al. 2011) and in studies with Parkinson's disease patients, who have compromised function in the basal ganglia (e.g., Shohamy et al. 2004).
An influential theory of reward-based learning has been the prediction error hypothesis, which stems from theories of how errors can shape associative connections (Rescorla and Wagner 1972) and temporal-difference reinforcement learning models (Sutton and Barto 1981). Specifically, this hypothesis posits that the neural circuitry of reward has the ability to update the expectation of future rewards and subsequently allow for the adaptation of behavior (Schultz 2002).
A prediction error can be characterized as the calculation of whether a reward is better or worse than expected (Glimcher 2011). A positive prediction error is generated when an unexpected reward occurs, leading to an increase in phasic firing of dopaminergic cells in the midbrain (Bayer and Glimcher 2005; Schultz et al. 1997). In contrast, a negative prediction error is recorded when an expected reward fails to occur. Although there is some debate whether the tonic firing rate of dopamine neurons makes it difficult to encode a negative prediction error (Bayer and Glimcher 2005), there is nonetheless depression of dopaminergic firing during the omission of an expected reward (Schultz et al. 1997). Both positive and negative prediction error signals are correlated to reward-evoked dopamine release onto the ventral striatum (Hart et al. 2014). These dopamine neurons show sensitivity to the temporal aspect of reward delivery, which corresponds to a key feature of the prediction error signal, a temporal learning element that allows for predictions about future rewards to be formulated and updated (Hollerman and Schultz 1998; Kobayashi and Schultz 2008; Roesch et al. 2007). Collectively, these findings and others point to dopamine as a key neural signal involved in signaling prediction errors.
In humans, a few fMRI studies have also reported dopaminergic midbrain activation during the generation of reward prediction errors (D'Ardenne et al. 2008, 2013). However, most have found evidence of a reward prediction signal in the striatum (for review, see Garrison et al. 2013). Some of the first observations of this involved simple comparisons of unexpected juice delivery (positive prediction error) and omission (negative prediction error), which evoked activation in dorsal (McClure et al. 2003; O'Doherty et al. 2004) and ventral (Berns et al. 2001; Gläscher et al. 2010; O'Doherty et al. 2003) striatum. These were soon followed by other studies demonstrating how such learning signals in the striatum could correlate with efficacious learning and performance (e.g., Schönberg et al. 2007).
In parallel with the reward prediction error hypothesis, reward-based reinforcement learning has also been demonstrated to involve two dissociable but related processes: one that encodes response-outcome associations to govern goal-directed behaviors, and the other that characterizes stimulus-response association to drive habitual behaviors (Balleine and O'Doherty 2010). Concurrent with studies in rodents, these two processes have been shown to also involve the human striatum (for review, see Dolan and Dayan 2013). To further illustrate how the striatum encodes habitual and goal-directed action selection, investigators have utilized computational models to capture the performance of these behaviors. For instance, a model-free approach is contingent upon the interaction between the learner and the reward stimulus to update the reward cue values through trial and error while reinforcing successful actions in a habitual manner (Balleine et al. 2008; Rangel et al. 2008). This approach supports neurophysiological data from dopamine (Bayer and Glimcher 2005; Schultz et al. 1997) and striatal neurons (Oyama et al. 2010; Stalnaker et al. 2012) and BOLD signal from the striatum (Garrison et al. 2013), hence drawing a parallel with the prediction error hypothesis. On the contrary, a model-based learning scheme encompasses a more flexible way of incorporating striatal prediction error signals into the calculation of value to inform goal-directed decision making (Dayan and Berridge 2014). This approach takes into account additional information about the expected reward, such as sensory attributes or associated costs (Doll et al. 2012), to allow the learner to form a “state-dependent” prediction error that encompasses the surrounding environment to drive goal-directed reward-maximizing actions (Gläscher et al. 2010). This state prediction error is dependent on not only the striatum, but also significant contributions from several cortical areas, such as lateral prefrontal cortex (Gläscher et al. 2010).
For both model-free and model-based approaches, the striatum might very well be the site where these two approaches are integrated to facilitate reward-based learning (Daw et al. 2011; Wunderlich et al. 2012), yet the underlying mechanism of how the striatum (and its distinct subsections) encodes reward prediction error has not been fully resolved (e.g., see study by Stenner et al. 2015). A recent multimodal study employing both positron emission tomography (PET) and fMRI reported that dopamine level in the ventral striatum is responsible for regulating the balance between model-free and model-based control on reward-related behavior (Deserno et al. 2015), further suggesting that the importance of dopaminergic modulation on the striatum cannot be discounted in either learning mechanism.
In short, these fMRI-based learning models demonstrate that the neural mechanism underpinning reward processing relies on diverse brain regions that interact with the striatum. Further progress in understanding how this reward-processing neural circuit encodes reward-related functions in humans will be contingent upon capitalizing on the many advantages that fMRI supplies, which will be scrutinized in subsequent sections.
The Promise of fMRI in Advancing Models of Reward Processing
As previously discussed, fMRI is a noninvasive way to study the human brain that provides us with correlative measurements of neural activity to allow for inferences in various affective and cognitive processes. We have focused thus far on how fMRI has confirmed prior findings from nonhuman studies and extended the knowledge to behaviors typically observed in humans. In this section, we now discuss advantages of a neuroimaging approach that have the potential to significantly advance models of reward processing.
Individual differences.
Due to its relative ease in application, fMRI studies have the potential to utilize relative large samples of subjects. Researchers can exploit these large samples by relating variation in brain structure and function to variation in behavior across individuals (Braver et al. 2010; Yarkoni and Braver 2010). While this approach can be problematic in underpowered studies (Yarkoni 2009), it provides a unique opportunity to identify candidate mechanisms that contribute to a range of psychological constructs (Braver et al. 2010; Hariri 2009).
Interindividual variability is often discussed in terms of structural and behavioral differences. Structural differences, which can be commonly detected using methods such as voxel-based morphometry from anatomical MRI images (Ashburner and Friston 2000; Good et al. 2001) and fractional anisotropy from diffusion tensor imaging (Jbabdi et al. 2015; Johansen-Berg and Behrens 2013), have been observed within both control population and pathological subgroups (Barrós-Loscertales et al. 2011; Pantelis et al. 2005; Thompson et al. 2001; Wright et al. 2000). These anatomical differences in gray matter volume and white matter integrity have been linked to interindividual behavioral differences (Kanai and Rees 2011), which includes measures such as reaction time (Jensen 1992), variable trait sensitivity to reward (Van den Berg et al. 2011) and working memory (Just and Carpenter 1992).
The link between neural anatomy and behavioral manifestation can be bridged by the functional interindividual variability, which stems from differences in neural responses recorded by fMRI. For example, fMRI studies looking at anhedonia, defined as the impaired capacity to experience pleasure (Treadway and Zald 2013), have found that increasing trait anhedonia not only correlated with reduced NAcc and caudate volume, but also with decreasing NAcc response to rewarding outcomes (Harvey et al. 2007; Wacker et al. 2009). In the same vein, fMRI studies investigating trait measures such as sensitivity to reward (Davis et al. 2004; Franken and Muris 2005) and behavioral indexes such as learning aptitude have been reported to correlate with striatal activation in response to reward anticipation (Beaver et al. 2006; Carter et al. 2009) and reward outcomes (Rieckmann et al. 2010; Schönberg et al. 2007). In addition, responses in striatum are predictive of individual differences in relative motivation to obtain different rewards (Clithero et al. 2011) and differences in strategic preferences (Venkatraman et al. 2009). These findings have been extended to patient populations where trait impulsivity (Chamorro et al. 2012; Cloninger et al. 1994) correlated with hyporesponsiveness in the ventral striatum during reward anticipation in both individuals with attention-deficit/hyperactivity disorder (Plichta and Scheres 2014) and detoxified alcoholics (Beck et al. 2009). Taken together, these findings suggest that interindividual behavioral variability to rewards is intricately tied to variations in striatum neural function.
These fMRI observations provided new translational opportunities to extend these findings to patient populations to predict susceptibility to psychopathologies. Linking behavioral differences with neural functional differences has major implications on the diagnosis of many psychopathologies and their individualized treatments. One example is a study by Telzer and colleagues (2014), where ventral striatal activation in adolescents exhibiting greater prosocial behaviors (e.g., donate money to family members) predicted longitudinal declines in depressive symptoms. In contrast, ventral striatal activation in adolescents who engaged in more selfish and risky reward-seeking behaviors predicted longitudinal increases in depressive symptoms (Telzer et al. 2014). Yet another example of how behavioral differences are associated with neural functional differences in psychopathologies is shown by Hanson and colleagues (2015a), who demonstrated that early life stress during childhood and adolescence, which leads to increased anxiety and depression (Norman et al. 2012), predicted diminished reward-related ventral striatal activity in adulthood. Collectively, these studies highlight how fMRI can be used to understand variations across individuals, which can be precursors of psychopathological conditions.
Brain connectivity and functional integration.
Much of the work that was discussed in the preceding sections is predicated on the principle of functional segregation, which relates functions (e.g., reward-related) to populations of neurons or single brain regions (e.g., striatum; Friston 2005; Raichle 2003). Yet, given the diverse anatomical inputs to each brain region, there can be multiple functions associated with such regions, making it difficult to understand how specific brain regions contribute to behavior and individual differences (Friston 2005; Park and Friston 2013). Addressing this issue rests with our ability to quantify the interactions and connectivity between brain regions, a principle known as functional integration (Friston 2009). Characterizing functional integration thus requires simultaneous measurements of responses from multiple brain regions, a core feature of neuroimaging studies. Indeed, one of the earliest neuroimaging studies reported functional connectivity (e.g., statistical dependencies or correlations) between homologous cortical areas (Biswal et al. 1995). More recent studies employing functional connectivity have provided remarkable insights into the large-scale network architecture of the brain (Beckmann et al. 2005; Smith et al. 2009). These networks span multiple regions and are recapitulated across species. For example, the default-mode network, which includes medial portions of the prefrontal cortex, posterior cingulate cortex, and lateral parts parietal cortex (Raichle et al. 2001), has been reported in rodents (Lu et al. 2012) and monkeys (Vincent et al. 2007). The ubiquity of large-scale networks has sparked several studies examining their functional significance and impact on behavior. These studies have demonstrated that functional connectivity with networks is associated with phenotypic variation (Ingalhalikar et al. 2014; Smith et al. 2014b) and behavioral variation (Cole et al. 2010; Smith et al. 2015) across individuals. In addition, functional connectivity with networks is tied to psychopathology, particularly depression (Berman et al. 2011) and schizophrenia (Manoliu et al. 2014). These studies highlight how neuroimaging can leverage functional connectivity to gain insight into the organization and functional significance of neural networks.
Beyond examining large-scale neural networks, functional connectivity has also been applied to the striatum in an effort to characterize connections with the reward circuit. For example, a landmark neuroimaging study with data from 1,000 participants utilized functional connectivity to reveal five striatal zones linked to sensorimotor, premotor, limbic, and two association networks (Choi et al. 2012), thus providing an in vivo characterization of careful tract-tracing studies performed in monkeys (Haber 2003). Recent neuroimaging work has added to these observations by quantifying how distinct cortical regions (e.g., orbitofrontal, dorsolateral, and parietal cortexes) converge on similar parts of the striatum (Jarbo and Verstynen 2015), supporting the hublike organization of striatal anatomical projections (Averbeck et al. 2014). Although corticostriatal interactions are important for reward processing, the striatum also interacts with midbrain nuclei, namely the substantia nigra and ventral tegmental area (VTA) (Haber and Knutson 2010). In accordance, a recent neuroimaging study developed a probabilistic atlas of the substantia nigra and VTA, allowing the authors to identify distinct patterns of functional connectivity with the striatum and cortical regions (Murty et al. 2014). The functional connections with the striatum have been exploited in a host of other studies, with several groups reporting disrupted corticostriatal interactions in social anxiety disorder (Manning et al. 2015), adolescent depression and anhedonia (Gabbay et al. 2013), and major depression and positive affect (Heller et al. 2013). Together, these observations reveal the interconnected nature of the striatum and underscore the importance of examining functional connectivity with the striatum.
Yet neurophysiologists have long recognized that functional connectivity suffers from critical limitations that preclude insight into neuronal coupling (Gerstein and Perkel 1969). Correlations between regions and variations in those correlations may be epiphenomenal, stemming from factors that are unrelated to neuronal coupling, such as changes in another connection, observational noise, or neuronal fluctuations (Friston 2011). To ameliorate these issues, neuroscientists have developed computational approaches that estimate effective connectivity (Friston 2011; Friston et al. 1997; Valdes-Sosa et al. 2011), which has revealed key insights into how interactions with the striatum shape reward processing. Unlike functional connectivity, studies using effective connectivity quantify how one region contributes to the observed signal within another region, according to a specific psychological context. These studies have broadened our understanding of how the striatum and its interconnected regions shape reward processing. For example, Kahnt and colleagues (2009) reported that, when participants computed reward prediction errors, dorsal striatum and ventral striatum were connected to the substantia nigra and VTA, respectively. Strikingly, the contribution of dorsal striatum to the observed signal within substantia nigra predicted the impact of different reinforcement types on subsequent behavior (Kahnt et al. 2009).
Other work using effective connectivity has revealed the interplay between different neural structures and striatal systems during reward processing. For instance, some studies have demonstrated that stimulus generalization during learning is mediated by striatal contributions to the hippocampal response (Kahnt et al. 2012; Wimmer et al. 2012). Studies using effective connectivity have also shown that hippocampal contributions to striatal responses play a role in value-based decision making (Wimmer and Shohamy 2012) and episodic memory encoding (Wimmer et al. 2014). Recent work has built on these observations by revealing how acute stress exacerbates ventromedial prefrontal contributions to the striatum (Maier et al. 2015) and striatal contributions to the amygdala (Admon et al. 2015). Although these studies highlight key patterns of effective connectivity with the striatum, we emphasize that these relationships should not be interpreted as causal; such inferences are difficult within fMRI (Ramsey et al. 2010) and likely require causal modeling approaches (Friston et al. 2003) combined with faster imaging protocols (Feinberg et al. 2010).
These studies underscore the importance of using fMRI to investigate brain connectivity and functional integration, concepts that are central to our understanding of how the striatum contributes to reward processing. We believe that future work has the potential to integrate effective and functional connectivity with structural connectivity. Indeed, structural connectivity with the striatum predicts personality characteristics (Cohen et al. 2009), such as recent observations of dissociable fiber tracts, leading to the striatum being associated with individual differences in temporal discounting (van den Bos et al. 2014). These findings raise important new questions regarding the convergence and divergence of various forms of brain connectivity (Adachi et al. 2012; Honey et al. 2010). Answering these questions will further elucidate the role of the striatum as part of a larger and dynamic reward circuit.
Multimodal approach using fMRI.
When used in isolation, fMRI, like all measurement techniques (e.g., single-unit recordings), are inherently correlational and descriptive (Rorden and Karnath 2004; Smith and Clithero 2009). This limitation can be partially overcome with the application of multimodal approaches, combining cellular-based techniques (e.g., neurophysiological recordings) and neurotransmitter-based techniques (e.g., PET) with fMRI, to inform on the neural basis of fMRI-measured brain activity. The integration across modalities is gaining traction in the study of reward processing in particular. For example, researchers have been relating fMRI findings to PET results in both meta-analysis and empirical studies to investigate how striatal BOLD signal is associated with dopamine release during reward-related behavior (Heinz et al. 2004; Judenhofer et al. 2008; Schott et al. 2008), thereby informing the underlying neuronal basis of the hemodynamic response. Efforts have also been expended to combine neurophysiological methods with fMRI in an attempt to link neural hemodynamic responses (fMRI) with the brain's canonical electrophysiological responses (Bland et al. 2011; Lee 2012). For example, simultaneous application of electroencephalography and fMRI demonstrated that the event-related potential signal correlated with the BOLD signals in the ventral striatum during the delivery of rewarding outcomes (Carlson et al. 2014; Carlson et al. 2011; Foti et al. 2014), suggesting a convergence of neurophysiological and hemodynamic signals. In addition, one recent study successfully applied optogenetics with fMRI in an animal model to characterize how stimulation of the VTA produced activation in the ventral striatum that shaped reward-related behavior (Ferenczi et al. 2016), providing further insights to understand the discrepancies (e.g., temporal resolution and cellular basis) between hemodynamic and neurophysiological measures. Collectively, studies integrating fMRI with other tools not only endows us with a deeper cellular-level understanding of the hemodynamic signal in fMRI (Goense and Logothetis 2008; Hayden and Platt 2011; Heeger and Ress 2002; Logothetis et al. 2001), but they also attribute fMRI findings in reward processing with potential cellular explanations.
Translational models of reward processing will ultimately require multimodal approaches that complement the strengths of fMRI, without compromising any of its inherent advantages (e.g., widespread noninvasive application in the human population). Such multimodal approaches call for the inclusion of noninvasive brain stimulation tools [e.g., transcranial magnetic stimulation (TMS), transcranial electrical stimulation (tES)] to task-based fMRI investigations (Poldrack and Farah 2015). This conjunction permits the transient manipulation of neural activity during task conditions to allow researchers to causally link brain stimulation to fMRI-measured neural alterations and resulting behavioral changes (Driver et al. 2009). The concurrent use of TMS and tES with fMRI has received recent attention in the cognitive neuroscience community (Antal et al. 2011; Blankenburg et al. 2008; Jang et al. 2009; Rushworth et al. 2002; Sack et al. 2007). Specifically, one recent study has successfully implemented transcranial alternating current stimulation, a form of temporally-precise tES (Helfrich et al. 2014), to demonstrate that intact frontal-parietal connectivity is necessary for value-based decision making in humans (Polanía et al. 2015). Despite the relative success of such TMS/tES-induced neural stimulation, there are preexisting hurdles left to overcome, such as the regional specificity of stimulation (Paulus 2011; Walsh and Cowey 2000). Nevertheless, the co-application of TMS/tES and fMRI is promising because it provides a means to causally link context-dependent neural activity with behavior (Camprodon and Halko 2014; Saiote et al. 2013).
Extending these multimodal approaches to study reward processing in humans remains challenging. For example, noninvasive brain stimulation approaches (e.g., tES) cannot directly (or selectively) access deep-brain structures like the striatum (Wagner et al. 2007). In contrast, invasive brain stimulation techniques (e.g., deep brain stimulation) that can access the striatum are often too invasive to be extensively applied in human participants. Therefore, one potential remedy that noninvasive multimodal studies in humans can exploit is to capitalize on the functional integration in the reward circuit to target the striatum and other deep-brain structures indirectly via their cortical connections. Application of tES to the prefrontal cortex, for example, alters connectivity with reward regions such as VTA (Chib et al. 2013) and striatum (Polanía et al. 2012). Similar work has also demonstrated that tES administered to prefrontal areas, including dorsolateral prefrontal cortex, implicates reward-related behaviors such as risk-taking (Sela et al. 2012), probabilistic learning (Turi et al. 2015), and social perception of unfair rewards (Knoch et al. 2008). The next step for these tES studies is to employ fMRI simultaneously with cortical brain stimulation to assess the responses of the striatum and other neural regions, so as to inform on the functional integration in the reward circuit. These types of multimodal studies will provide an exciting opportunity to expand our knowledge on reward processing within the human brain, potentially providing the gateway to developing brain-stimulation-based therapeutic interventions for a host of psychopathologies.
Conclusions, Limitations, and Future Considerations
With the widespread application of fMRI, influential nonhuman animal findings on the role of the striatum in reward processing have been successfully corroborated in both healthy and patient human populations. Many fMRI studies have also broadened the understanding of reward processing in the striatum to human attributes, such as distinctly human incentives (e.g., money) and social and environmental contexts more representative of human society. As fMRI matures into a powerful cognitive neuroscience tool, increased effort has been expended to use fMRI to investigate individual differences in neural functions, which can potentially explain the link between behavioral variability and susceptibility to psychopathologies. Moreover, greater emphasis on brain connectivity and functional integration may help refine existing neural models of reward processing. Brain connectivity findings could potentially be combined with noninvasive brain stimulation to draw causal inferences regarding the mechanistic links between corticostriatal pathways and reward. Collectively, these advancements in applying fMRI (Fig. 4) promise translational opportunities that can inform on the diagnostic and therapeutic insights of many psychopathologies.
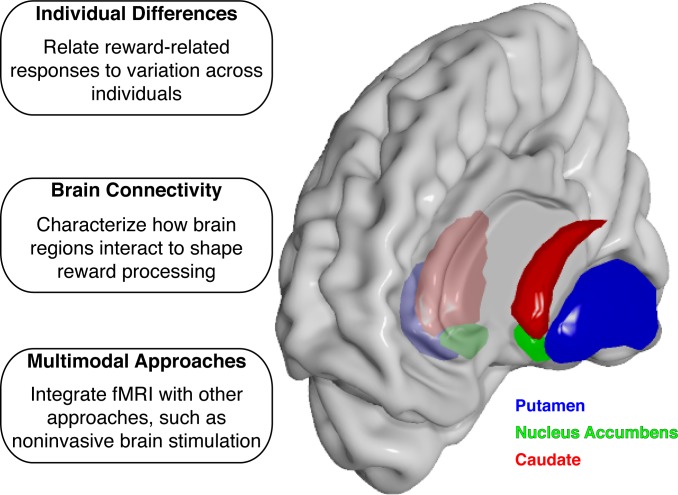
Fig. 4.
The promise of fMRI in understanding reward processing. Shown here is an anterior view of a translucent cortical surface for the right hemisphere. Bilateral striatal surfaces are shown for the putamen (blue), NAcc (green), and caudate (red). Our synthesis of the literature suggests that fMRI holds promise for understanding individual differences and brain connectivity. In addition, multimodal approaches that combine fMRI with other tools, such as noninvasive brain stimulation, may reveal causal mechanisms that support reward processing. Brain surfaces were created with Chris Rorden's Surf Ice software.
Nevertheless, there are limitations on what fMRI can accomplish for translational research. One notable limitation is that individual difference studies require a larger sample than those typically recruited for fMRI experiments (Button et al. 2013; Yarkoni et al. 2011). Furthermore, variables within these large samples may interact (e.g., age and race). The development of a population-based atlas can help mitigate this concern as it aims to capture interindividual variability and map functional cortical organization that can be broadly applied in individuals across different groups (Wang et al. 2015a). Such continued future efforts to maximize the exploration of individual differences will play an important role in explaining behavioral variability that informs clinical preventive and diagnostic applications (Poldrack and Farah 2015).
Another potential source of limitation of applying fMRI to translational research is the difficulty of some fMRI-based functional integration analysis in drawing causal inferences on neural connectivity. Without the capability to demonstrate directionality in neural connectivity, it is challenging to develop effective target-specific treatment and preventive measures. This barrier has been partially overcome with dynamic causal modeling, which was shown to be reliable in making causal interpretations (Smith et al. 2011). Yet another shortcoming in the current fMRI literature is the flexibility in data analysis procedures, with preprocessing and analytic options rivaling the number of fMRI studies (Carp 2012). The practice of standardizing experimental reporting guidelines in journal publications is gaining traction in the field (Poldrack et al. 2008), which will yield greater transparency in both experimental design and analytic approaches, as well as improve the reproducibility of fMRI findings (Poldrack and Poline 2015).
Despite these limitations, fMRI has generated some interesting directions that will help shape future research on cognitive and affective processes such as reward processing.
First, fMRI studies have began to explore the neural basis of many psychological constructs that are inherent to the human reward processing mechanism. For example, the loss of voluntary control in decision making (Haggard 2008), which is pertinent to many maladaptive reward approach and consummatory behaviors (Bechara 2005; Volkow et al. 2011), has been studied with presence and absence of choices (Ernst et al. 2004; Leotti and Delgado 2011), habitual reward-based learning (Tricomi et al. 2009), controllable and uncontrollable setbacks to goal-directed reward-seeking behavior (Bhanji and Delgado 2014), and compulsive reward-seeking and reward-taking behavior in addiction (e.g., food: Gearhardt et al. 2011; cocaine: Tomasi et al. 2015). Future studies will benefit from examining whether individual differences in behavioral variability (e.g., impulsivity) are predictive of the loss of voluntary control and how the neural connectivity is altered during this maladaptive decision making, using fMRI-centric multimodal approaches. Furthermore, future studies can also take advantage of brain connectivity to clarify and augment knowledge about how neural circuits, beyond a particular region of interest, may contribute to a psychological process. For instance, recent work has leveraged brain connectivity to distinguish representations tied to distinct properties of reward, particularly those related to affect (e.g., pleasure) and those related to information (e.g., reinforcement), to show that these properties are not distinguishable at the region of interest level, but instead can emerge as a function of connectivity between corticostriatal circuits (Smith et al. 2016).
Second, the application of computational models to fMRI, such as those that gave rise to model-free and model-based learning mechanisms, have opened the door for new translational opportunities (Montague et al. 2012; Stephan et al. 2015; Wang and Krystal 2014). These new opportunities will revolve around using neural computational mechanisms to predict behavior and understand its adaptive consequences, which could have both diagnostic and prognostic values. Perhaps more importantly, the successful application of computational models may serve to bridge findings from diverse techniques while connecting animal models with human data (Bornkessel-Schlesewsky et al. 2015; Kepecs and Mainen 2012).
Third, improvements in fMRI acquisition (e.g., three-dimensional or multiplex echo planar imaging: Feinberg et al. 2010; finer-resolution fMRI: Yacoub et al. 2015) may help elucidate functional segregation within the striatum, such as dissociating the functional role of NAcc core and shell in the human brain, which is currently not well-characterized in humans (Baliki et al. 2013). At present, there remains some technical obstacles to overcome for the acquisition of excellent subcortical signals, such as those within striatal subregions (Kaza et al. 2011; Polanía et al. 2015). Nevertheless, the progress in refining fMRI technical capabilities will greatly enhance the capacity to use fMRI to study functional dissociation within smaller human subcortical subregions (e.g., striatum), while also improving the ability to detect BOLD activation (Iranpour et al. 2015; Posse et al. 2012).
Although some scholars have questioned the utility of using neuroimaging to understand behavioral phenomena (Gul and Pesendorfer 2008), we contend that knowledge gained from neuroimaging studies can contribute to behavioral theories and potentially even impact policy (Clithero et al. 2008; Levallois et al. 2012; Venkatraman 2013). This approach has been observed in some reward-related studies. For example, neural estimates of reward have been used to optimize public goods allocation and solve the pernicious problem of free riders (Krajbich et al. 2009), while a novel theory of overbidding during auctions, e.g., loss contemplation, rather than risk aversion, was developed and tested based on reward-related responses observed in the striatum (Delgado et al. 2008). More recent studies have used neural data to access individual preferences in the absence of choices (Smith et al. 2014a) and to adjudicate between disparate theories of investor behavior (Frydman et al. 2014). These are just some examples that illustrate how neuroimaging can inform our understanding of behavior and policy.
Together, these new research avenues congregate on the fundamental notion that fMRI is a crucial and promising tool to study cognitive and affective processing in humans. Advancements in the study of these processes hinge on profiting from the advantages of fMRI, while simultaneously implementing complementary tools, such as brain stimulation, to make causal inferences on neural functions and circuitry connectivity. This multimodal approach will endow us with a deeper and more comprehensive understanding of mechanistic underpinnings to these cognitive and affective processes and also provide the translational basis for both therapeutic and preventive healthcare measures.
GRANTS
This study was funded by National Institutes of Health Grants R01-DA027764 (to M. R. Delgado) and F32-MH107175 (to D. V. Smith).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.S.W., D.V.S., and M.R.D. conception and design of research; K.S.W., D.V.S., and M.R.D. drafted manuscript; K.S.W., D.V.S., and M.R.D. edited and revised manuscript; K.S.W., D.V.S., and M.R.D. approved final version of manuscript; D.V.S. prepared figures.
ACKNOWLEDGMENTS
We thank Eunbin S. Kim and Heena Manglani for helpful comments on previous drafts of the manuscript, and Dominic Fareri for assistance with a figure.
REFERENCES
- Adachi Y, Osada T, Sporns O, Watanabe T, Matsui T, Miyamoto K, Miyashita Y. Functional connectivity between anatomically unconnected areas is shaped by collective network-level effects in the macaque cortex. Cereb Cortex 22: 1586–1592, 2012. [DOI] [PubMed] [Google Scholar]
- Admon R, Holsen LM, Aizley H, Remington A, Whitfield-Gabrieli S, Goldstein JM, Pizzagalli DA. Striatal hypersensitivity during stress in remitted individuals with recurrent depression. Biol Psychiatry 78: 67–76, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal A, Polania R, Schmidt-Samoa C, Dechent P, Paulus W. Transcranial direct current stimulation over the primary motor cortex during fMRI. Neuroimage 55: 590–596, 2011. [DOI] [PubMed] [Google Scholar]
- Apicella P, Ljungberg T, Scarnati E, Schultz W. Responses to reward in monkey dorsal and ventral striatum. Exp Brain Res 85: 491–500, 1991. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry-the methods. Neuroimage 11: 805–821, 2000. [DOI] [PubMed] [Google Scholar]
- Averbeck BB, Lehman J, Jacobson M, Haber SN. Estimates of projection overlap and zones of convergence within frontal-striatal circuits. J Neurosci 34: 9497–9505, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Mansour A, Baria AT, Huang L, Berger SE, Fields HL, Apkarian AV. Parceling human accumbens into putative core and shell dissociates encoding of values for reward and pain. J Neurosci 33: 16383–16393, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Daw ND, O'Doherty JP. Multiple forms of value learning and the function of dopamine. In: Neuroeconomics: Decision Making and the Brain, edited by Glimcher PW, Camerer CF, Fehr E, Poldrack RA. Boston, MA: Elsevier, 2008, p. 367–385. [Google Scholar]
- Balleine BW, O'Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35: 48–69, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn Reson Med 25: 390–397, 1992. [DOI] [PubMed] [Google Scholar]
- Barrós-Loscertales A, Garavan H, Bustamante JC, Ventura-Campos N, Llopis JJ, Belloch V, Parcet MA, Ávila C. Reduced striatal volume in cocaine-dependent patients. Neuroimage 56: 1021–1026, 2011. [DOI] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage 76: 412–427, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer HM, Glimcher PW. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron 47: 129–141, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. J Neurosci 26: 5160–5166, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci 8: 1458–1463, 2005. [DOI] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wüstenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hägele C, Knutson B, Heinz A. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry 66: 734–742, 2009. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360: 1001–1013, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens P, Logothetis NK, Tolias AS. Local field potentials, BOLD and spiking activity-relationships and physiological mechanisms. Nat Prec 5216.1, 2010. [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Soc Cogn Affect Neurosci 6: 548–555, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns GS, McClure SM, Pagnoni G, Montague PR. Predictability modulates human brain response to reward. J Neurosci 21: 2793–2798, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci 26: 507–513, 2003. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev 28: 309–369, 1998. [DOI] [PubMed] [Google Scholar]
- Bhanji JP, Delgado MR. Perceived control influences neural responses to setbacks and promotes persistence. Neuron 83: 1369–1375, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop M, Elder ST, Heath RG. Intracranial self-stimulation in man. Science 140: 394–396, 1963. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34: 537–541, 1995. [DOI] [PubMed] [Google Scholar]
- Bland AR, Mushtaq F, Smith DV. Exploiting trial-to-trial variability in multimodal experiments. Front Hum Neurosci 5: 80, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenburg F, Ruff CC, Bestmann S, Bjoertomt O, Eshel N, Josephs O, Weiskopf N, Driver J. Interhemispheric effect of parietal TMS on somatosensory response confirmed directly with concurrent TMS-fMRI. J Neurosci 28: 13202–13208, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornkessel-Schlesewsky I, Schlesewsky M, Small SL, Rauschecker JP. Neurobiological roots of language in primate audition: common computational properties. Trends Cogn Sci 19: 142–150, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Cole MW, Yarkoni T. Vive les differences! Individual variation in neural mechanisms of executive control. Curr Opin Neurobiol 20: 242–250, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP. Acute effects of cocaine on human brain activity and emotion. Neuron 19: 591–611, 1997. [DOI] [PubMed] [Google Scholar]
- Brown PL, Jenkins HM. Auto-shaping of the pigeon's key-peck. J Exp Anal Behav 11: 1–8, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafò MR. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14: 365–376, 2013. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Frank LR. A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation. J Cereb Blood Flow Metab 17: 64–72, 1997. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludağ K, Dubowitz DJ, Liu TT. Modeling the hemodynamic response to brain activation. Neuroimage 23: S220–S233, 2004. [DOI] [PubMed] [Google Scholar]
- Camprodon JA, Halko MA. Combination of transcranial magnetic stimulation (TMS) with functional magnetic resonance imaging. In: Transcranial Magnetic Stimulation. New York: Springer, 2014, p. 179–196. [Google Scholar]
- Carlson JM, Foti D, Harmon-Jones E, Proudfit GH. Midbrain volume predicts fMRI and ERP measures of reward reactivity. Brain Struct Funct 220: 1861–1866, 2014. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G. Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. Neuroimage 57: 1608–1616, 2011. [DOI] [PubMed] [Google Scholar]
- Carp J. The secret lives of experiments: methods reporting in the fMRI literature. Neuroimage 63: 289–300, 2012. [DOI] [PubMed] [Google Scholar]
- Carter RM, MacInnes JJ, Huettel SA, Adcock RA. Activation in the VTA and nucleus accumbens increases in anticipation of both gains and losses. Front Behav Neurosci 3: 21, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro J, Bernardi S, Potenza MN, Grant JE, Marsh R, Wang S, Blanco C. Impulsivity in the general population: a national study. J Psychiatr Res 46: 994–1001, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albert D, O'Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain's reward circuitry. Dev Sci 14: F1–F10, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chib VS, Yun K, Takahashi H, Shimojo S. Noninvasive remote activation of the ventral midbrain by transcranial direct current stimulation of prefrontal cortex. Transl Psychiatry 3: e268, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EY, Yeo BT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol 108: 2242–2263, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumbley J, Tobler P, Fehr E. Fatal attraction: Ventral striatum predicts costly choice errors in humans. Neuroimage 89: 1–9, 2014. [DOI] [PubMed] [Google Scholar]
- Clithero JA, Rangel A. Informatic parcellation of the network involved in the computation of subjective value. Soc Cogn Affect Neurosci 9: 1289–1302, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero JA, Reeck C, Carter RM, Smith DV, Huettel SA. Nucleus accumbens mediates relative motivation for rewards in the absence of choice. Front Hum Neurosci 5: 87, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero JA, Tankersley D, Huettel SA. Foundations of neuroeconomics: from philosophy to practice. PLoS Biol 6: e298, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR, Przybeck TR, Svrakic DM. The Temperament and Character Inventory (TCI): A Guide to Its Development and Use. St. Louis, MO: Center for Psychobiology of Personality, Washington University, 1994. [Google Scholar]
- Cohen MX, Schoene-Bake JC, Elger CE, Weber B. Connectivity-based segregation of the human striatum predicts personality characteristics. Nat Neurosci 12: 32–34, 2009. [DOI] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. Neuroimage 52: 590–599, 2010. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Schultz W. Effects of expectations for different reward magnitudes on neuronal activity in primate striatum. J Neurophysiol 89: 2823–2838, 2003. [DOI] [PubMed] [Google Scholar]
- D'Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science 319: 1264–1267, 2008. [DOI] [PubMed] [Google Scholar]
- D'Ardenne K, Lohrenz T, Bartley KA, Montague PR. Computational heterogeneity in the human mesencephalic dopamine system. Cogn Affect Behav Neurosci 13: 747–756, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Strachan S, Berkson M. Sensitivity to reward: implications for overeating and overweight. Appetite 42: 131–138, 2004. [DOI] [PubMed] [Google Scholar]
- Daw ND, Doya K. The computational neurobiology of learning and reward. Curr Opin Neurobiol 16: 199–204, 2006. [DOI] [PubMed] [Google Scholar]
- Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ. Model-based influences on humans' choices and striatal prediction errors. Neuron 69: 1204–1215, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P, Berridge KC. Model-based and model-free Pavlovian reward learning: revaluation, revision, and revelation. Cogn Affect Behav Neurosci 14: 473–492, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M, Stenger V, Fiez J. Motivation-dependent responses in the human caudate nucleus. Cereb Cortex 14: 1022–1030, 2004. [DOI] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Ann N Y Acad Sci 1104: 70–88, 2007. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll D, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol 84: 3072–3077, 2000. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Schotter A, Ozbay EY, Phelps EA. Understanding overbidding: using the neural circuitry of reward to design economic auctions. Science 321: 1849–1852, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci 32: 5549–5552, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deserno L, Huys QJ, Boehme R, Buchert R, Heinze HJ, Grace AA, Dolan RJ, Heinz A, Schlagenhauf F. Ventral striatal dopamine reflects behavioral and neural signatures of model-based control during sequential decision making. Proc Natl Acad Sci U S A 112: 1595–1600, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of Pavlovian approach behavior. J Neurosci 21: 9471–9477, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson KC, Li J, Delgado MR. Parallel contributions of distinct human memory systems during probabilistic learning. Neuroimage 55: 266–276, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ, Dayan P. Goals and habits in the brain. Neuron 80: 312–325, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll BB, Simon DA, Daw ND. The ubiquity of model-based reinforcement learning. Curr Opin Neurobiol 22: 1075–1081, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver J, Blankenburg F, Bestmann S, Vanduffel W, Ruff CC. Concurrent brain-stimulation and neuroimaging for studies of cognition. Trends Cogn Sci 13: 319–327, 2009. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, Zarahn E, Leibenluft E, Zametkin A, Towbin K. Choice selection and reward anticipation: an fMRI study. Neuropsychologia 42: 1585–1597, 2004. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8: 1481–1489, 2005. [DOI] [PubMed] [Google Scholar]
- Fareri DS, Niznikiewicz MA, Lee VK, Delgado MR. Social network modulation of reward-related signals. J Neurosci 32: 9045–9052, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Gunther M, Glasser MF, Miller KL, Ugurbil K, Yacoub E. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PloS One 5: e15710, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D, Katovich K, Mehta H, Patenaude B, Ramakrishnan C, Kalanithi P, Etkin A, Knutson B, Glover GH, Deisseroth K. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science 351: 41–53, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald TH, Friston KJ, Dolan RJ. Action-specific value signals in reward-related regions of the human brain. J Neurosci 32: 16417–16423, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerde K, Shohamy D. Feedback timing modulates brain systems for learning in humans. J Neurosci 31: 13157–13167, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Carlson JM, Sauder CL, Proudfit GH. Reward dysfunction in major depression: multimodal neuroimaging evidence for refining the melancholic phenotype. Neuroimage 101: 50–58, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IH, Muris P. Individual differences in reward sensitivity are related to food craving and relative body weight in healthy women. Appetite 45: 198–201, 2005. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity: a review. Brain Connect 1: 13–36, 2011. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Modalities, modes, and models in functional neuroimaging. Science 326: 399–403, 2009. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Models of brain function in neuroimaging. Annu Rev Psychol 56: 57–87, 2005. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6: 218–229, 1997. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage 19: 1273–1302, 2003. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189–210, 1994. [Google Scholar]
- Frydman C, Barberis N, Camerer C, Bossaerts P, Rangel A. Using neural data to test a theory of investor behavior: An application to realization utility. J Finance 69: 907–946, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Ely BA, Li Q, Bangaru SD, Panzer AM, Alonso CM, Castellanos FX, Milham MP. Striatum-based circuitry of adolescent depression and anhedonia. J Am Acad Child Adolesc Psychiatry 52: 628–641, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison J, Erdeniz B, Done J. Prediction error in reinforcement learning: a meta-analysis of neuroimaging studies. Neurosci Biobehav Rev 37: 1297–1310, 2013. [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD. Neural correlates of food addiction. Arch Gen Psychiatry 68: 808–816, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein GL, Perkel DH. Simultaneously recorded trains of action potentials: analysis and functional interpretation. Science 164: 828–830, 1969. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Daw N, Dayan P, O'Doherty JP. States versus rewards: dissociable neural prediction error signals underlying model-based and model-free reinforcement learning. Neuron 66: 585–595, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glimcher PW. Understanding dopamine and reinforcement learning: the dopamine reward prediction error hypothesis. Proc Natl Acad Sci U S A 108: 15647–15654, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goense JB, Logothetis NK. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr Biol 18: 631–640, 2008. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Fristen K, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14: 21–36, 2001. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O'Doherty J, Dolan RJ. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. J Neurosci 22: 10829–10837, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gul F, Pesendorfer W. The case for mindless economics. In: The Foundations of Positive and Normative Economics. New York: Oxford University Press, 2008, p. 3–42. [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat 26: 317–330, 2003. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35: 4–26, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggard P. Human volition: towards a neuroscience of will. Nat Rev Neurosci 9: 934–946, 2008. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Albert WD, Iselin AMR, Carré JM, Dodge KA, Hariri AR. Cumulative stress in childhood is associated with blunted reward-related brain activity in adulthood. Soc Cogn Affect Neurosci nsv124, 2015a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hariri AR, Williamson DE. Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biol Psychiatry 78: 598–605, 2015b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annu Rev Neurosci 32: 225–247, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AS, Rutledge RB, Glimcher PW, Phillips PE. Phasic dopamine release in the rat nucleus accumbens symmetrically encodes a reward prediction error term. J Neurosci 34: 698–704, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey P, Pruessner J, Czechowska Y, Lepage M. Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Mol Psychiatry 12: 767–775, 2007. [DOI] [PubMed] [Google Scholar]
- Hassani OK, Cromwell HC, Schultz W. Influence of expectation of different rewards on behavior-related neuronal activity in the striatum. J Neurophysiol 85: 2477–2489, 2001. [DOI] [PubMed] [Google Scholar]
- Hayden BY, Platt ML. On the difficulties of integrating evidence from fMRI and electrophysiology in cognitive neuroscience. In: Decision Making, Affect, and Learning: Attention and Performance XXIII, edited by Delgado MR, Phelps EA, Robbins TW. New York: Oxford University Press, 2011, p. 125–152. [Google Scholar]
- Hayden BY, Smith DV, Platt ML. Electrophysiological correlates of default-mode processing in macaque posterior cingulate cortex. Proc Natl Acad Sci U S A 106: 5948–5953, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeger DJ, Ress D. What does fMRI tell us about neuronal activity? Nat Rev Neurosci 3: 142–151, 2002. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grüsser-Sinopoli SM, Flor H, Braus DF, Buchholz HG, Gründer G. Correlation between dopamine D2 receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry 161: 1783–1789, 2004. [DOI] [PubMed] [Google Scholar]
- Helfrich RF, Schneider TR, Rach S, Trautmann-Lengsfeld SA, Engel AK, Herrmann CS. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr Biol 24: 333–339, 2014. [DOI] [PubMed] [Google Scholar]
- Heller AS, Fox AS, Wing EK, McQuisition KM, Vack NJ, Davidson RJ. The neurodynamics of affect in the laboratory predicts persistence of real-world emotional responses. J Neurosci 35: 10503–10509, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, Johnstone T, Light SN, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Relationships between changes in sustained fronto-striatal connectivity and positive affect in major depression resulting from antidepressant treatment. Am J Psychiatry 170: 197–206, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Usui S. Functional properties of monkey caudate neurons. III. Activities related to expectation of target and reward. J Neurophysiol 61: 814–832, 1989. [DOI] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn Reson Med 42: 849–863, 1999. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci 1: 304–309, 1998. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Tremblay L, Schultz W. Influence of reward expectation on behavior-related neuronal activity in primate striatum. J Neurophysiol 80: 947–963, 1998. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Thivierge JP, Sporns O. Can structure predict function in the human brain? Neuroimage 52: 766–776, 2010. [DOI] [PubMed] [Google Scholar]
- Huber L, Goense J, Kennerley AJ, Ivanov D, Krieger SN, Lepsien J, Trampel R, Turner R, Möller HE. Investigation of the neurovascular coupling in positive and negative BOLD responses in human brain at 7T. Neuroimage 97: 349–362, 2014. [DOI] [PubMed] [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci U S A 111: 823–828, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iranpour J, Morrot G, Claise B, Jean B, Bonny JM. Using high spatial resolution to improve BOLD fMRI detection at 3T. PloS One 10: e0141358, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Doya K. Distinct neural representation in the dorsolateral, dorsomedial, and ventral parts of the striatum during fixed-and free-choice tasks. J Neurosci 35: 3499–3514, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron 58: 284–294, 2008. [DOI] [PubMed] [Google Scholar]
- Jang SH, Ahn SH, Byun WM, Kim CS, Lee MY, Kwon YH. The effect of transcranial direct current stimulation on the cortical activation by motor task in the human brain: an fMRI study. Neurosci Lett 460: 117–120, 2009. [DOI] [PubMed] [Google Scholar]
- Jankowski KF, Moore WE, Merchant JS, Kahn LE, Pfeifer JH. But do you think I'm cool?: Developmental differences in striatal recruitment during direct and reflected social self-evaluations. Dev Cogn Neurosci 8: 40–54, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbo K, Verstynen TD. Converging structural and functional connectivity of orbitofrontal, dorsolateral prefrontal, and posterior parietal cortex in the human striatum. J Neurosci 35: 3865–3878, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jbabdi S, Sotiropoulos SN, Haber SN, Van Essen DC, Behrens TE. Measuring macroscopic brain connections in vivo. Nat Neurosci 18: 1546–1555, 2015. [DOI] [PubMed] [Google Scholar]
- Jensen AR. The importance of intraindividual variation in reaction time. Pers Individ Dif 13: 869–881, 1992. [Google Scholar]
- Johansen-Berg H, Behrens TE. Diffusion MRI: From Quantitative Measurement to In Vivo Neuroanatomy. New York: Academic, 2013. [Google Scholar]
- Judenhofer MS, Wehrl HF, Newport DF, Catana C, Siegel SB, Becker M, Thielscher A, Kneilling M, Lichy MP, Eichner M. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat Med 14: 459–465, 2008. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA. A capacity theory of comprehension: individual differences in working memory. Psychol Rev 99: 122–149, 1992. [DOI] [PubMed] [Google Scholar]
- Kahnt T, Park SQ, Burke CJ, Tobler PN. How glitter relates to gold: similarity-dependent reward prediction errors in the human striatum. J Neurosci 32: 16521–16529, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahnt T, Park SQ, Cohen MX, Beck A, Heinz A, Wrase J. Dorsal striatal-midbrain connectivity in humans predicts how reinforcements are used to guide decisions. J Cogn Neurosci 21: 1332–1345, 2009. [DOI] [PubMed] [Google Scholar]
- Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nat Rev Neurosci 12: 231–242, 2011. [DOI] [PubMed] [Google Scholar]
- Kawagoe R, Takikawa Y, Hikosaka O. Expectation of reward modulates cognitive signals in the basal ganglia. Nat Neurosci 1: 411–416, 1998. [DOI] [PubMed] [Google Scholar]
- Kaza E, Klose U, Lotze M. Comparison of a 32-channel with a 12-channel head coil: are there relevant improvements for functional imaging? J Magn Reson Imaging 34: 173–183, 2011. [DOI] [PubMed] [Google Scholar]
- Kepecs A, Mainen ZF. A computational framework for the study of confidence in humans and animals. Philos Trans R Soc Lond B Biol Sci 367: 1322–1337, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R, Hyder F, Blumenfeld H. Physiological basis of BOLD fMRI decreases. In: Neurovascular Coupling Methods. New York: Springer, 2014, p. 221–236. [Google Scholar]
- Klein JT, Platt ML. Social information signaling by neurons in primate striatum. Curr Biol 23: 691–696, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingner CM, Hasler C, Brodoehl S, Witte OW. Dependence of the negative BOLD response on somatosensory stimulus intensity. Neuroimage 53: 189–195, 2010. [DOI] [PubMed] [Google Scholar]
- Knoch D, Nitsche MA, Fischbacher U, Eisenegger C, Pascual-Leone A, Fehr E. Studying the neurobiology of social interaction with transcranial direct current stimulation-the example of punishing unfairness. Cereb Cortex 18: 1987–1990, 2008. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21: RC159, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Wimmer GE, Kuhnen CM, Winkielman P. Nucleus accumbens activation mediates the influence of reward cues on financial risk taking. NeuroReport 19: 509–513, 2008. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Schultz W. Influence of reward delays on responses of dopamine neurons. J Neurosci 28: 7837–7846, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajbich I, Camerer C, Ledyard J, Rangel A. Using neural measures of economic value to solve the public goods free-rider problem. Science 326: 596–599, 2009. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci 12: 535–540, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron 47: 763–770, 2005. [DOI] [PubMed] [Google Scholar]
- Lacey S, Hagtvedt H, Patrick VM, Anderson A, Stilla R, Deshpande G, Hu X, Sato JR, Reddy S, Sathian K. Art for reward's sake: visual art recruits the ventral striatum. Neuroimage 55: 420–433, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau B, Glimcher PW. Action and outcome encoding in the primate caudate nucleus. J Neurosci 27: 14502–14514, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau B, Glimcher PW. Value representations in the primate striatum during matching behavior. Neuron 58: 451–463, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen M, Gold L. Brain function and neurophysiological correlates of signals used in functional neuroimaging. J Neurosci 23: 3972–3980, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH. Informing brain connectivity with optogenetic functional magnetic resonance imaging. Neuroimage 62: 2244–2249, 2012. [DOI] [PubMed] [Google Scholar]
- Leotti LA, Delgado MR. The inherent reward of choice. Psychol Sci 22: 1310–1318, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levallois C, Clithero JA, Wouters P, Smidts A, Huettel SA. Translating upwards: linking the neural and social sciences via neuroeconomics. Nat Rev Neurosci 13: 789–797, 2012. [DOI] [PubMed] [Google Scholar]
- Locurto C, Terrace H, Gibbon J. Autoshaping, random control, and omission training in the RAT1. J Exp Anal Behav 26: 451–462, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos Trans R Soc Lond B Biol Sci 357: 1003–1037, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. What we can do and what we cannot do with fMRI. Nature 453: 869–878, 2008. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Panzeri S. Local field potential, relationship to BOLD signal. In: Encyclopedia of Computational Neuroscience. New York: Springer, 2015, p. 1560–1568. [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157, 2001. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol 66: 735–769, 2004. [DOI] [PubMed] [Google Scholar]
- Lu H, Zou Q, Gu H, Raichle ME, Stein EA, Yang Y. Rat brains also have a default mode network. Proc Natl Acad Sci U S A 109: 3979–3984, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri C, Schridde U, Murayama Y, Panzeri S, Logothetis NK. The amplitude and timing of the BOLD signal reflects the relationship between local field potential power at different frequencies. J Neurosci 32: 1395–1407, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SU, Makwana AB, Hare TA. Acute stress impairs self-control in goal-directed choice by altering multiple functional connections within the brain's decision circuits. Neuron 87: 621–631, 2015. [DOI] [PubMed] [Google Scholar]
- Manning J, Reynolds G, Saygin ZM, Hofmann SG, Pollack M, Gabrieli JD, Whitfield-Gabrieli S. Altered resting-state functional connectivity of the frontal-striatal reward system in social anxiety disorder. PLoS One 10: e0125286, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A, Riedl V, Zherdin A, Mühlau M, Schwerthöffer D, Scherr M, Peters H, Zimmer C, Förstl H, Bäuml J, Wohlschläger AM, Sorg C. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr Bull 40: 428–437, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Cowen PJ, Harmer CJ. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol Psychiatry 67: 439–445, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron 38: 339–346, 2003. [DOI] [PubMed] [Google Scholar]
- McGinty VB, Lardeux S, Taha SA, Kim JJ, Nicola SM. Invigoration of reward seeking by cue and proximity encoding in the nucleus accumbens. Neuron 78: 910–922, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci 15: 394–408, 2003. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Rev 31: 236–250, 2000. [DOI] [PubMed] [Google Scholar]
- Montague PR, Dolan RJ, Friston KJ, Dayan P. Computational psychiatry. Trends Cogn Sci 16: 72–80, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullinger K, Mayhew S, Bagshaw A, Bowtell R, Francis S. Evidence that the negative BOLD response is neuronal in origin: A simultaneous EEG-BOLD-CBF study in humans. Neuroimage 94: 263–274, 2014. [DOI] [PubMed] [Google Scholar]
- Murty VP, Shermohammed M, Smith DV, Carter RM, Huettel SA, Adcock RA. Resting state networks distinguish human ventral tegmental area from substantia nigra. Neuroimage 100: 580–589, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara D, Ozaki N, Miura Y, Miura H, Nagatsu T. Increased dopamine and serotonin metabolism in rat nucleus accumbens produced by intracranial self-stimulation of medial forebrain bundle as measured by in vivo microdialysis. Brain Res 495: 178–181, 1989. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Forstmann BU, Wagenmakers EJ. Erroneous analyses of interactions in neuroscience: a problem of significance. Nat Neurosci 14: 1105–1107, 2011. [DOI] [PubMed] [Google Scholar]
- Nir Y, Fisch L, Mukamel R, Gelbard-Sagiv H, Arieli A, Fried I, Malach R. Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Curr Biol 17: 1275–1285, 2007. [DOI] [PubMed] [Google Scholar]
- Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T. The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Med 9: e1001349, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 304: 452–454, 2004. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron 38: 329–337, 2003. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron 33: 815–826, 2002. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A 89: 5951–5955, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds J. Pleasure centers in the brain. Sci Am 195: 105–117, 1956. [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol 47: 419–427, 1954. [DOI] [PubMed] [Google Scholar]
- Oyama K, Hernádi I, Iijima T, Tsutsui KI. Reward prediction error coding in dorsal striatal neurons. J Neurosci 30: 11447–11457, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelis C, Yücel M, Wood SJ, Velakoulis D, Sun D, Berger G, Stuart GW, Yung A, Phillips L, McGorry PD. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull 31: 672–696, 2005. [DOI] [PubMed] [Google Scholar]
- Park HJ, Friston K. Structural and functional brain networks: from connections to cognition. Science 342: 1238411, 2013. [DOI] [PubMed] [Google Scholar]
- Parkinson J, Dalley J, Cardinal R, Bamford A, Fehnert B, Lachenal G, Rudarakanchana N, Halkerston K, Robbins T, Everitt B. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behav Brain Res 137: 149–163, 2002. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive Pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity byd-amphetamine. J Neurosci 19: 2401–2411, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Willoughby PJ, Robbins TW, Everitt BJ. Disconnection of the anterior cingulate cortex and nucleus accumbens core impairs Pavlovian approach behavior: further evidence for limbic cortical-ventral striatopallidal systems. Behav Neurosci 114: 42–63, 2000. [PubMed] [Google Scholar]
- Pasley BN, Inglis BA, Freeman RD. Analysis of oxygen metabolism implies a neural origin for the negative BOLD response in human visual cortex. Neuroimage 36: 269–276, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus W. Transcranial electrical stimulation (tES-tDCS; tRNS, tACS) methods. Neuropsychol Rehabil 21: 602–617, 2011. [DOI] [PubMed] [Google Scholar]
- Peciña S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic “liking” for food: map based on microinjection Fos plumes. Brain Res 863: 71–86, 2000. [DOI] [PubMed] [Google Scholar]
- Peterson GB, Ackilt JE, Frommer GP, Hearst ES. Conditioned approach and contact behavior toward signals for food or brain-stimulation reinforcement. Science 177: 1009–1011, 1972. [DOI] [PubMed] [Google Scholar]
- Plichta MM, Scheres A. Ventral-striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci Biobehav Rev 38: 125–134, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanía R, Moisa M, Opitz A, Grueschow M, Ruff CC. The precision of value-based choices depends causally on fronto-parietal phase coupling. Nat Commun 6: 8090, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanía R, Paulus W, Nitsche MA. Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum Brain Mapp 33: 2499–2508, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Farah MJ. Progress and challenges in probing the human brain. Nature 526: 371–379, 2015. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Fletcher PC, Henson RN, Worsley KJ, Brett M, Nichols TE. Guidelines for reporting an fMRI study. Neuroimage 40: 409–414, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Mumford JA, Nichols TE. Handbook of Functional MRI Data Analysis. Cambridge, UK: University Press, 2011. [Google Scholar]
- Poldrack RA, Poline JB. The publication and reproducibility challenges of shared data. Trends Cogn Sci 19: 59–61, 2015. [DOI] [PubMed] [Google Scholar]
- Porcelli AJ, Lewis AH, Delgado MR. Acute stress influences neural circuits of reward processing. Front Neurosci 6: 157, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R, Conrad D, Brady J. Some neural and behavioral correlates of electrical self-stimulation of the limbic system. J Exp Anal Behav 2: 43–55, 1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posse S, Ackley E, Mutihac R, Rick J, Shane M, Murray-Krezan C, Zaitsev M, Speck O. Enhancement of temporal resolution and BOLD sensitivity in real-time fMRI using multi-slab echo-volumar imaging. Neuroimage 61: 115–130, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. Functional brain imaging and human brain function. J Neurosci 23: 3959–3962, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Grubb RL, Gado MH, Eichling JO, Ter-Pogossian MM. Correlation between regional cerebral blood flow and oxidative metabolism: in vivo studies in man. Arch Neurol 33: 523–526, 1976. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A 98: 676–682, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey JD, Hanson SJ, Hanson C, Halchenko YO, Poldrack RA, Glymour C. Six problems for causal inference from fMRI. Neuroimage 49: 1545–1558, 2010. [DOI] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci 9: 545–556, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch A, Rainer G, Logothetis NK. The effect of a serotonin-induced dissociation between spiking and perisynaptic activity on BOLD functional MRI. Proc Natl Acad Sci U S A 105: 6759–6764, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G, Friston K, Koch C. A direct quantitative relationship between the functional properties of human and macaque V5. Nat Neurosci 3: 716–723, 2000. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Classical Conditioning: Current Research and Theory. New York: Appleton-Century-Crofts, 1972, p. 64–99. [Google Scholar]
- Rieckmann A, Fischer H, Bäckman L. Activation in striatum and medial temporal lobe during sequence learning in younger and older adults: relations to performance. Neuroimage 50: 1303–1312, 2010. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol 6: 228–236, 1996. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Calu DJ, Schoenbaum G. Dopamine neurons encode the better option in rats deciding between differently delayed or sized rewards. Nat Neurosci 10: 1615–1624, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath HO. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat Rev Neurosci 5: 812–819, 2004. [DOI] [PubMed] [Google Scholar]
- Rushworth M, Hadland K, Paus T, Sipila P. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol 87: 2577–2592, 2002. [DOI] [PubMed] [Google Scholar]
- Sack AT, Kohler A, Bestmann S, Linden DE, Dechent P, Goebel R, Baudewig J. Imaging the brain activity changes underlying impaired visuospatial judgments: simultaneous FMRI, TMS, and behavioral studies. Cereb Cortex 17: 2841–2852, 2007. [DOI] [PubMed] [Google Scholar]
- Saiote C, Turi Z, Paulus W, Antal A. Combining functional magnetic resonance imaging with transcranial electrical stimulation. Front Hum Neurosci 7: 435, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima K, Ueda Y, Doya K, Kimura M. Representation of action-specific reward values in the striatum. Science 310: 1337–1340, 2005. [DOI] [PubMed] [Google Scholar]
- Schönberg T, Daw ND, Joel D, O'Doherty JP. Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward-based decision making. J Neurosci 27: 12860–12867, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Seidenbecher CI, Coenen HH, Heinze HJ, Zilles K. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci 28: 14311–14319, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schridde U, Khubchandani M, Motelow JE, Sanganahalli BG, Hyder F, Blumenfeld H. Negative BOLD with large increases in neuronal activity. Cereb Cortex 18: 1814–1827, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol 57: 87–115, 2006. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron 36: 241–263, 2002. [DOI] [PubMed] [Google Scholar]
- Schultz W. Neuronal reward and decision signals: from theories to data. Physiol Rev 95: 853–951, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T, Romo R, Scarnati E. Reward-related activity in the monkey striatum and substantia nigra. Prog Brain Res 99: 227–235, 1993. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci 12: 4595–4610, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science 275: 1593–1599, 1997. [DOI] [PubMed] [Google Scholar]
- Sela T, Kilim A, Lavidor M. Transcranial alternating current stimulation increases risk-taking behavior in the balloon analog risk task. Front Neurosci 6: 22, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci 9: 569–577, 2006. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Myers C, Onlaor S, Gluck M. Role of the basal ganglia in category learning: how do patients with Parkinson's disease learn? Behav Neurosci 118: 676–686, 2004. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL, Hyder F. A BOLD search for baseline. Neuroimage 36: 277–281, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Li C. Imaging stress-and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev 26: 25–31, 2007. [DOI] [PubMed] [Google Scholar]
- Smith A, Bernheim BD, Camerer CF, Rangel A. Neural activity reveals preferences without choices. Am Econ J Microecon 6: 1–36, 2014a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Clithero JA. Manipulating executive function with transcranial direct current stimulation. Front Integr Neurosci 3: 26, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Delgado MR. Reward processing. In: Brain Mapping: An Encyclopedic Reference, edited by Toga AW, Poldrack RA. Waltham, MA: Academic Press, 2015, p. 361–366. [Google Scholar]
- Smith DV, Hayden BY, Truong TK, Song AW, Platt ML, Huettel SA. Distinct value signals in anterior and posterior ventromedial prefrontal cortex. J Neurosci 30: 2490–2495, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Rigney AE, Delgado MR. Distinct reward properties are encoded via corticostriatal interactions. Sci Rep 6: 20093, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Sip KE, Delgado MR. Functional connectivity with distinct neural networks tracks fluctuations in gain/loss framing susceptibility. Hum Brain Mapp 36: 2743–2755, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DV, Utevsky AV, Bland AR, Clement N, Clithero JA, Harsch AE, Carter RM, Huettel SA. Characterizing individual differences in functional connectivity using dual-regression and seed-based approaches. Neuroimage 95: 1–12, 2014b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A 106: 13040–13045, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Miller KL, Salimi-Khorshidi G, Webster M, Beckmann CF, Nichols TE, Ramsey JD, Woolrich MW. Network modelling methods for FMRI. Neuroimage 54: 875–891, 2011. [DOI] [PubMed] [Google Scholar]
- Speer ME, Bhanji JP, Delgado MR. Savoring the past: positive memories evoke value representations in the striatum. Neuron 84: 847–856, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalnaker TA, Calhoon GG, Ogawa M, Roesch MR, Schoenbaum G. Reward prediction error signaling in posterior dorsomedial striatum is action specific. J Neurosci 32: 10296–10305, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenner MP, Rutledge RB, Zaehle T, Schmitt FC, Kopitzki K, Kowski AB, Voges J, Heinze HJ, Dolan RJ. No unified reward prediction error in local field potentials from the human nucleus accumbens: evidence from epilepsy patients. J Neurophysiol 114: 781–792, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Iglesias S, Heinzle J, Diaconescu AO. Translational perspectives for computational neuroimaging. Neuron 87: 716–732, 2015. [DOI] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Reinforcement Learning: An Introduction. Cambridge, MA: MIT Press, 1998. [Google Scholar]
- Sutton RS, Barto AG. Toward a modern theory of adaptive networks: expectation and prediction. Psychol Rev 88: 135–170, 1981. [PubMed] [Google Scholar]
- Tai LH, Lee AM, Benavidez N, Bonci A, Wilbrecht L. Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value. Nat Neurosci 15: 1281–1289, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir DI, Mitchell JP. Disclosing information about the self is intrinsically rewarding. Proc Natl Acad Sci U S A 109: 8038–8043, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Galván A. Neural sensitivity to eudaimonic and hedonic rewards differentially predict adolescent depressive symptoms over time. Proc Natl Acad Sci U S A 111: 6600–6605, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, Van Erp T, Poutanen VP, Huttunen M, Lönnqvist J, Standertskjöld-Nordenstam CG, Kaprio J, Khaledy M. Genetic influences on brain structure. Nat Neurosci 4: 1253–1258, 2001. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Wang GJ, Wang R, Caparelli EC, Logan J, Volkow ND. Overlapping patterns of brain activation to food and cocaine cues in cocaine abusers. Hum Brain Mapp 36: 120–136, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Parsing anhedonia translational models of reward-processing deficits in psychopathology. Curr Dir Psychol Sci 22: 244–249, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi E, Balleine BW, O'Doherty JP. A specific role for posterior dorsolateral striatum in human habit learning. Eur J Neurosci 29: 2225–2232, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi E, Fiez JA. Feedback signals in the caudate reflect goal achievement on a declarative memory task. Neuroimage 41: 1154–1167, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron 41: 281–292, 2004. [DOI] [PubMed] [Google Scholar]
- Turi Z, Mittner M, Opitz A, Popkes M, Paulus W, Antal A. Transcranial direct current stimulation over the left prefrontal cortex increases randomness of choice in instrumental learning. Cortex 63: 145–154, 2015. [DOI] [PubMed] [Google Scholar]
- Valdes-Sosa PA, Roebroeck A, Daunizeau J, Friston K. Effective connectivity: Influence, causality and biophysical modeling. Neuroimage 58: 339–361, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg I, Franken IH, Muris P. Individual differences in sensitivity to reward. J Psychophysiol 25: 81–86, 2011. [Google Scholar]
- van den Bos W, Rodriguez CA, Schweitzer JB, McClure SM. Connectivity strength of dissociable striatal tracts predict individual differences in temporal discounting. J Neurosci 34: 10298–10310, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman V. Why bother with the brain? A role for decision neuroscience in understanding strategic variability. Prog Brain Res 202: 267–288, 2013. [DOI] [PubMed] [Google Scholar]
- Venkatraman V, Huettel SA, Chuah LY, Payne JW, Chee MW. Sleep deprivation biases the neural mechanisms underlying economic preferences. J Neurosci 31: 3712–3718, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman V, Payne JW, Bettman JR, Luce MF, Huettel SA. Separate neural mechanisms underlie choices and strategic preferences in risky decision making. Neuron 62: 593–602, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447: 83–86, 2007. [DOI] [PubMed] [Google Scholar]
- Viswanathan A, Freeman RD. Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat Neurosci 10: 1308–1312, 2007. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A 108: 15037–15042, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage 46: 327–337, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade AR. The negative BOLD signal unmasked. Neuron 36: 993–995, 2002. [DOI] [PubMed] [Google Scholar]
- Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng 9: 527–565, 2007. [DOI] [PubMed] [Google Scholar]
- Walsh V, Cowey A. Transcranial magnetic stimulation and cognitive neuroscience. Nat Rev Neurosci 1: 73–80, 2000. [DOI] [PubMed] [Google Scholar]
- Wang D, Buckner RL, Fox MD, Holt DJ, Holmes AJ, Stoecklein S, Langs G, Pan R, Qian T, Li K, Baker JT, Stufflebeam SM, Wang K, Wang X, Hong B, Liu H. Parcellating cortical functional networks in individuals. Nat Neurosci 18: 1853–1860, 2015a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KS, McClure JP Jr, Alselehdar SK, Kanta V. Direct and indirect pathways of the basal ganglia: opponents or collaborators? Front Neuroanat 9: 20, 2015b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Krystal JH. Computational psychiatry. Neuron 84: 638–654, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Williams H. Auto-maintenance in the pigeon: sustained pecking despite contingent non-reinforcement. J Exp Anal Behav 12: 511–520, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer GE, Braun EK, Daw ND, Shohamy D. Episodic memory encoding interferes with reward learning and decreases striatal prediction errors. J Neurosci 34: 14901–14912, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer GE, Daw ND, Shohamy D. Generalization of value in reinforcement learning by humans. Eur J Neurosci 35: 1092–1104, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer GE, Shohamy D. Preference by association: how memory mechanisms in the hippocampus bias decisions. Science 338: 270–273, 2012. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited-again. Neuroimage 2: 173–181, 1995. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry 157: 16–25, 2000. [DOI] [PubMed] [Google Scholar]
- Wunderlich K, Dayan P, Dolan RJ. Mapping value based planning and extensively trained choice in the human brain. Nat Neurosci 15: 786–791, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub E, Harel N, Shmuel A. High-resolution fMRI. In: fMRI: From Nuclear Spins to Brain Functions. New York: Springer, 2015, p. 769–791. [Google Scholar]
- Yarkoni T. Big correlations in little studies: inflated fMRI correlations reflect low statistical power–commentary on Vul et al. (2009). Perspect Psychol Sci 4: 294–298, 2009. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Braver TS. Cognitive neuroscience approaches to individual differences in working memory and executive control: Conceptual and methodological issues, edited by Gruszka A, Matthews G, Szymura B. In: Handbook of Individual Differences in Cognition. New York: Springer, 2010, p. 87–107. [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 8: 665–670, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]