Mimoun et al. were first to describe a peculiar yellowish pattern in patients with age-related macular degeneration (AMD) as reticular pseudodrusen (RPD) in 1990.1 Since that time, improved identification and clinical descriptions of RPD and their dynamic nature have become possible with the development of newer imaging modalities including infrared and spectral domain optical coherence tomography (SD-OCT) imaging. By most, RPD are considered to be associated with or at least have some developmental relationship to subretinal drusenoid deposits (SDD), which have been identified histopathologically as residing internal to the retinal pigment epithelium (RPE).2 Immunohistochemistry and confocal microscopy studies revealed that SDD are composed of similar molecules as soft drusen, but the lack of opsins suggests that the material has not been derived from photoreceptor outer segments.3
Associations have been identified between RPD and polymorphisms in Age Related Maculopathy Susceptibility 2 (ARMS2), female gender, impairments in dark adaptation, and choroidal thinning. Recent evidence supports the presence of RPD as a risk factor for the development and progression of geographic atrophy (GA) and the development of neovascular disease in the fellow eye in patients with wet AMD. Our study investigates if patients with advanced AMD - (GA) and/or choroidal neovascularization (CNV) - are more likely to have RPD than patients with early stages of AMD or no AMD.
A retrospective review of fundus photos, spectral domain optical coherence tomography (Spectralis SD-OCT; Heidelberg Engineering, Heidelberg, Germany or Cirrus HD-OCT; Carl Zeiss Meditec, Dublin, CA), infrared imaging (Spectralis, Heidelberg), and fundus autofluorescence (Spectralis, Heidelberg) images of 1178 patients was performed by two of the authors (JLK and SGS). Patients had been recruited from Bascom Palmer Eye Institute and had been evaluated for AMD and assigned a modified AREDS grade (1–5) based on AMD severity (Table 1). It was not possible to mask the 2 authors to the AREDS grade during image review. This study was approved by the Institutional Review Board at the University of Miami Miller School of Medicine, was compliant with the Health Insurance Portability and Accountability Act of 1996, adhered to the tenets of the Declaration of Helsinki.
Table 1.
Fundus Photograph Grading System
| Grade | Description |
| 1 | No drusen or small nonextensive drusen, without pigment abnormalities |
| 2 | Extensive small drusen or nonextensive intermediate drusen or pigment abnormalities associated with AMD |
| 3 | Extensive intermediate drusen or any large drusen |
| 4 | GA, with or without involvement of the center of the macula |
| 5 | Exudative AMD, including nondrusenoid pigment epithelial detachments, serous or hemorrhagic retinal detachments, subretinal or subRPE hemorrhage or fibrosis, or photocoagulation scars consistent with treatment of AMD |
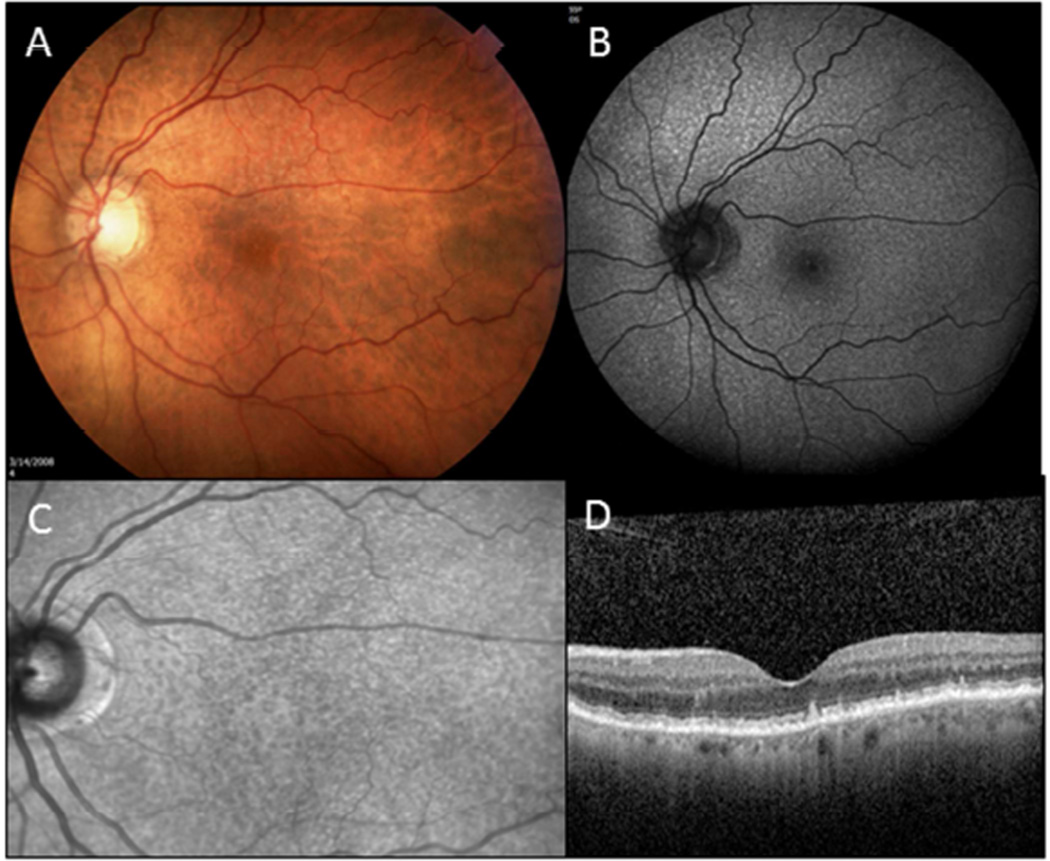
Multimodal imaging review of 1178 patients revealed that 197 (17%) had reticular pseudodrusen (Figure 1). One hundred and six (18%) of the 570 patients with CNV had reticular pseudodrusen and 30 (22%) of the 136 patients with GA had reticular pseudodrusen. Sixty-one (13%) of the 472 patients with early or no AMD (grade 1 – 3) had RPD on imaging review. A chi-squared test was used to determine whether there was a difference between AMD grade and the presence of reticular pseudodrusen.
Figure 1.
Reticular pseudodrusen (RPD) shown in different imaging modalities. (A) Color fundus photography displays RPD as yellowish-pale lesions in the mostly in the superior macula and extending superior to the macula. (B) Fundus autofluorescence (Spectralis, Heidelberg) image reveals diffuse hyperfluorescent lesions. (C) Infrared reflectance (Spectralis, Heidelberg) image demonstrates hyporeflective lesions. (D) SD-OCT (Spectralis, Heidelberg) shows various stages of subretinal drusenoid deposits (SDD).
Statistical analysis demonstrated a significant association between AMD severity and the presence of reticular pseudodrusen (p=0.01). Nineteen percent of patients with GA and/or CNV had reticular pseudodrusen versus 13% in those with early or no AMD. Patients with advanced AMD had higher odds of reticular pseudodrusen (GA, odds ratio (OR)=1.9; CNV, OR=1.5) compared with those with early stages of AMD or no AMD.
This study provides further evidence in support of a relationship between reticular pseudodrusen and advanced AMD. It has also been suggested that RPD could progress into a distinct form of late AMD, given that gradual disappearance of RPD and regression of SDD have been associated with outer retinal atrophy in the absence of typical GA.4 As we begin to appreciate the potential effect of RPD on the natural history of AMD progression, identification of RPD for patient stratification and future treatment decisions has growing importance. Considering their small size, confluent reticular pattern, and tendency to spread beyond the macula, identification of RPD can be challenging. Currently, a multimodal imaging approach is favored, especially including examination of SD-OCT, infrared imaging, and fundus autofluoresence imaging.5
Supplementary Material
Acknowledgments
Funding was provided by NIH center grant 2R01EY012118-11 and by an unrestricted grant to the University of Miami from Research to Prevent Blindness, New York, NY. The funding organization had no role in the design or conduct of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
American Academy of Ophthalmology Annual Meeting, 2015
The authors have no conflict of interest with the material presented in this article.
References
- 1.Mimoun G, Soubrane G, Coscas G. Macular drusen [in French] J Fr Ophthalmol. 1990;13:511–530. [PubMed] [Google Scholar]
- 2.Spaide RF, Curcio CA. Drusen characterization with multimodal imaging. Retina. 2010;30:1441–1454. doi: 10.1097/IAE.0b013e3181ee5ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oak AS, Messinger JD, Curcio CA. Subretinal drusenoid deposits: further characterization by lipid histochemistry. Retina. 2014;34:825–826. doi: 10.1097/IAE.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 4.Spaide RF. Outer retinal atrophy after regression of subretinal drusenoid deposits as a newly recognized form of late age-related macular degeneration. Retina. 2013;33(9):1800–1808. doi: 10.1097/IAE.0b013e31829c3765. [DOI] [PubMed] [Google Scholar]
- 5.De Bats F, Mathis T, Mauget-Faysse M, et al. Prevalence of reticular pseudodrusen in age-related macular degeneration using multimodal imaging. Retina. 2015 doi: 10.1097/IAE.0000000000000648. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.