Table 3.
Acceptor substrate specificities of β4GalTs using a sequential two-step process.a
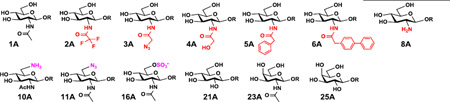 | ||||||
|---|---|---|---|---|---|---|
| # | Acceptor | β3GlcNAcT* | NmLgtB | Hpβ4GalT | Bβ4GalT | |
| 1A | GlcNAcβ1–3LacβMU | 90–95% | NmLgtA | 100% | 100% | 90–95% |
| 2A | GlcNTFAβ1–3LacβMU | 100% | NmLgtA | 95–100% | 100% | 60–65% |
| 3A | GlcNAcN3β1–3LacβMU | 25–35% | NmLgtA | 100% | 100% | 35–40% |
| 4A | GlcNGcβ1–3LacβMU | 55–60% | Hpβ3GlcNAcT | 90–95% | 75–80% | 0 |
| 5A | GlcNAcPhβ1–3LacβMU | 20–25% | NmLgtA | 100% | 50% | 20–25% |
| 6A | GlcNAcPhPhβ1–3LacβMU | <5% | NmLgtA | 100% | 0 | 0 |
| 8A | GlcNH2β1–3LacβMU | 20–25% | NmLgtA | 0 | 0 | 0 |
| 10A | GlcNAc6NH2β1–3LacβMU | 70–75% | Hpβ3GlcNAcT | 0 | 20% | 0 |
| 11A | GlcNAc6N3β1–3LacβMU | 30–35% | Hpβ3GlcNAcT | 5–10% | 100% | 0 |
| 16A | GlcNAc6Sβ1–3LacβMU | 65–70% | Hpβ3GlcNAcT | 0 | 100% | 0 |
| 21A | Glcβ1–3LacβMU | 20–25% | NmLgtA | 95–100% | 0 | 0 |
| 23A | GalNAcβ1–3LacβMU | 80–90% | NmLgtA | 0 | 0 | 0 |
| 25A | Galβ1–3LacβMU | 40–45% | NmLgtA | 0 | 0 | 0 |
Yields estimated by HRMS. R= LacβMU.
LacβMU was used as the acceptor for β3GlcNAcT-catalyzed reactions. The trisaccharide producing reactions with a higher yield (catalyzed by either Hpβ3GlcNAcT or NmLgtA) were used for the acceptor substrate specificity studies of β-4GalTs.
