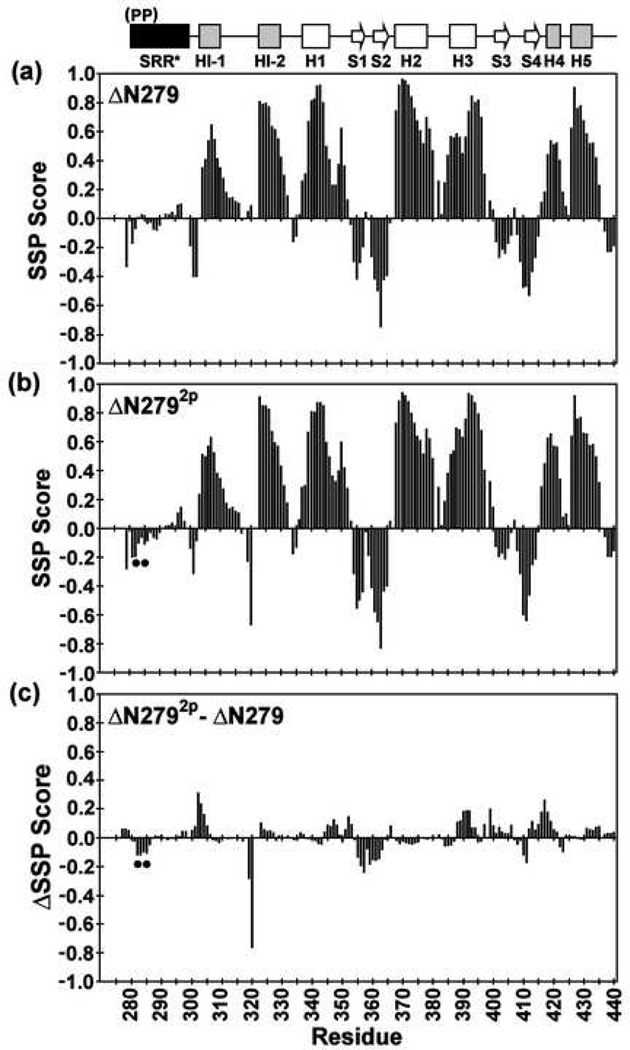
Fig. 3.
The SRR* of ΔN279 and ΔN2792p lack any predominant secondary structure, and phosphorylation does not alter the secondary structure of the inhibitory module or ETS domain. Shown are the secondary structural propensity (SSP) scores for (a) ΔN279 and (b) ΔN2792p, along with the (c) change ΔSSP upon phosphorylation, derived from available main chain 1H, 13C, and 15N chemical shifts (and using random coil phosphoserine chemical shifts25). Values approaching +1 and −1 are indicative of well defined α-helices and β-strands, respectively24. The phosphoserines are indicated by dots, and missing data points correspond to residues with unassigned NMR signals.